-
PDF
- Split View
-
Views
-
Cite
Cite
Bart Larsen, Timothy D Verstynen, Fang-Cheng Yeh, Beatriz Luna, Developmental Changes in the Integration of Affective and Cognitive Corticostriatal Pathways are Associated with Reward-Driven Behavior, Cerebral Cortex, Volume 28, Issue 8, August 2018, Pages 2834–2845, https://doi.org/10.1093/cercor/bhx162
Close - Share Icon Share
Abstract
The relative influence of affective and cognitive processes on behavior is increasingly understood to transform through development, from adolescence into adulthood, but the neuroanatomical mechanisms underlying this change are not well understood. We analyzed diffusion magnetic resonance imaging in 115 10- to 28-year-old participants to identify convergent corticostriatal projections from cortical systems involved in affect and cognitive control and determined the age-related differences in their relative structural integrity. Results indicate that the relative integrity of affective projections, in relation to projections from cognitive control systems, decreases with age and is positively associated with reward-driven task performance. Together, these findings provide new evidence that developmental differences in the integration of corticostriatal networks involved in affect and cognitive control underlie known developmental decreases in the propensity for reward-driven behavior into adulthood.
Introduction
Adolescence is a unique stage of development characterized, in part, by increases in reward-driven behavior that, while adaptive in nature, can lead to maladaptive risk-taking that undermines survival (Shulman et al. 2016). Developmental cognitive neuroscience models propose that this adolescent predisposition is driven by a unique balance between the influence of systems supporting affective processes, including socioemotional and reward processing (i.e., limbic systems), and systems supporting cognitive control on behavior, with affective systems having a greater relative influence on behavior in adolescence than in adulthood (Shulman et al. 2016). These influential models have been generated in large part from human neuroimaging observations that suggest unique maturational trajectories for brain areas commonly ascribed to cognitive control (e.g., prefrontal and related circuitry) and incentive processing (e.g., ventral striatum and cortical limbic circuitry). Structural magnetic resonance imaging (MRI) studies indicate protracted maturation of brain regions involved in in reward processing and cognitive control (e.g., prefrontal cortex, striatum) from adolescence to adulthood into adulthood with unique timelines (Giedd et al. 1999; Sowell et al. 1999; Gogtay et al. 2004; Mills et al. 2014). Relatedly, functional MRI (fMRI) studies suggest differential task-related activation of brain regions involved in in both systems from adolescence to adulthood (Geier and Luna 2009; van Leijenhorst et al. 2010; Bari and Robbins 2013). Evidence for age-related changes in the functional integration of these systems is more limited. Graph theoretical analyses of large-scale structural and functional network organization indicate developmental enhancements in the global integration of systems and networks including subcortical and frontal systems (Dennis et al. 2013; Hwang et al. 2013; Baker et al. 2015; Marek et al. 2015); however, developmental changes in the specific functional integration of systems involved in limbic and cognitive control functions, and their association with reward-driven behavior, have not been probed directly. This limits our ability to understand the interactive dynamics underlying the relative contributions of these systems to behavior.
An ideal target for investigating developmental shifts in the influence of cortical brain systems on behavior is corticostriatal circuitry. The striatum is the primary input nucleus to the basal ganglia and functions to bias action selection (Humphries et al. 2006; Houk et al. 2007; Kimchi and Laubach 2009). It is neuroanatomically well-positioned for this function, receiving dense projections from the cerebral cortex, including cortical brain systems involved in affective and cognitive control processes (Alexander et al. 1986; Choi et al. 2012). The striatum has long been thought to integrate cortical information within closed, parallel circuits, but more recently human (Verstynen et al. 2012; Verstynen 2014; Jarbo and Verstynen 2015) and nonhuman primate (Averbeck et al. 2014; Choi et al. 2016) studies have shown that areas of the striatum receive convergent projections from functionally disparate cortical regions. These convergent zones are thought to serve as functional hubs that directly integrate and synchronize information to drive basal ganglia action outputs (Haber 2003, 2014; Averbeck et al. 2014). Convergent projections from limbic and cognitive control-related cortical systems into the striatum then represent an important neuroanatomical substrate for the integration of affective and executive information to influence behavior. Further, the striatum has been shown to structurally develop into adulthood (Sowell et al. 1999; Larsen and Luna 2014; Raznahan et al. 2014) and play a critical role in increasing global network integration during adolescence (Marek et al. 2015). The development of convergent corticostriatal inputs from limbic and cognitive control-related systems may thus be an important developmental mechanism for the changing relative influence of cognitive control and limbic functional brain systems on adolescent behavior.
Here we analyze diffusion MRI data to characterize the relative integrity of convergent corticostriatal projections from cortical systems functionally involved in affect processing (i.e., limbic networks) and cognitive control (i.e., frontal-parietal and attention networks) (Yeo et al. 2011) and determine the nature of these convergent inputs changes across development. Specifically, we hypothesized that projections from predominantly affective cortical systems into striatal convergent zones would have greater relative integrity than projections from predominantly cognitive control-related cortical systems early in adolescence, with the affective influence decreasing into adulthood, and the nature of this convergence relationship would be associated with individual differences to reward-driven behavior.
Materials and Methods
Sample
A total of 115 adolescents and young-adults between the ages of 10 and 28 participated in this study (M = 18.43, standard deviation (SD) = 4.67; 62 males). Eighteen participants were excluded due to either excess head motion during the diffusion MRI (dMRI) scan, low temporal signal-to-noise ratio (tSNR), or visible distortion or artifact in the raw diffusion images (details described below). All participants were recruited from the community and reported no history of neurological disease or brain injury and no personal history or first-degree relative with major psychiatric illness. A description of the sample can be found in Table 1. All experimental procedures in this study complied with the Code of Ethics of the World Medical Association (1964 Declaration of Helsinki) and the Institutional Review Board at the University of Pittsburgh. Participants gave informed consent and were paid for their participation in the study. Aspects of this data have been previously reported in studies of resting-state network development (Hwang et al. 2013) and incentive processing (Paulsen et al. 2014).
Sample demographics
| Variable . | Mean (SD) or count . | Range . |
|---|---|---|
| Age | 18.43 (4.67) | 10–28 |
| Sex | 62 M/53 F | |
| Race | 79 W; 17 B; 10 A; 9 O | |
| Mother education | 5.75 (1.15) | 3–7 |
| Father education | 5.58 (1.13) | 3–7 |
| IQ | 114.6 (12.7) | 76–138 |
| Variable . | Mean (SD) or count . | Range . |
|---|---|---|
| Age | 18.43 (4.67) | 10–28 |
| Sex | 62 M/53 F | |
| Race | 79 W; 17 B; 10 A; 9 O | |
| Mother education | 5.75 (1.15) | 3–7 |
| Father education | 5.58 (1.13) | 3–7 |
| IQ | 114.6 (12.7) | 76–138 |
Note. M = male, F = female; W = white, B = black, A = Asian, O = other (multiple endorsements = 7, not endorsed = 2); education levels are: 1 = less than 7th grade, 2 = junior high school, 3 = partial high school, 4 = completed high school or equivalent, 5 = some college, 6 = completed college, 7 = completed postgraduate training. Four participants did not indicate mother’s education and 5 participants did not indicate father’s education.
Sample demographics
| Variable . | Mean (SD) or count . | Range . |
|---|---|---|
| Age | 18.43 (4.67) | 10–28 |
| Sex | 62 M/53 F | |
| Race | 79 W; 17 B; 10 A; 9 O | |
| Mother education | 5.75 (1.15) | 3–7 |
| Father education | 5.58 (1.13) | 3–7 |
| IQ | 114.6 (12.7) | 76–138 |
| Variable . | Mean (SD) or count . | Range . |
|---|---|---|
| Age | 18.43 (4.67) | 10–28 |
| Sex | 62 M/53 F | |
| Race | 79 W; 17 B; 10 A; 9 O | |
| Mother education | 5.75 (1.15) | 3–7 |
| Father education | 5.58 (1.13) | 3–7 |
| IQ | 114.6 (12.7) | 76–138 |
Note. M = male, F = female; W = white, B = black, A = Asian, O = other (multiple endorsements = 7, not endorsed = 2); education levels are: 1 = less than 7th grade, 2 = junior high school, 3 = partial high school, 4 = completed high school or equivalent, 5 = some college, 6 = completed college, 7 = completed postgraduate training. Four participants did not indicate mother’s education and 5 participants did not indicate father’s education.
dMRI Acquisition
Imaging data were collected using a 3.0 T Trio (Siemens) scanner at the Magnetic Resonance Research Center (MRRC), Presbyterian University Hospital, Pittsburgh, PA. The images were acquired with a total of 60 diffusion sampling directions (repetition time = 6.4 s, echo time = 0.89 s, field of view = 255 × 255 mm, 52 slices) and a single-shell b-value of 850 s/mm2. Two b = 0 images were collected. The in-plane resolution was 2.5 mm. The slice thickness was 2.5 mm. Participants viewed a movie of their choosing for the duration of the acquisition.
dMRI Preprocessing
Eddy current and motion correction were performed using the “eddy” function (Andersson and Sotiropoulos 2016) from the FMRIB Software Library (FSL; Jenkinson et al. 2012). Participants with motion estimates that exceeded 2.5 SDs above the sample mean were excluded from further analyses. Motion metrics (and means after exclusion and cutoffs) were the following: mean volume-by-volume translation (M = 0.35 mm, cutoff = 0.85 mm) and rotation (M = 0.35 mm, cutoff = 0.95 mm) and percentage of slices with signal dropout (e.g., Benner et al. 2011; Yendiki et al. 2014) (M = 0.41%, cutoff = 3.5%). For the remaining participants, these metrics were used as continuous covariates in all subsequent statistical analyses. Participants were also excluded if their tSNR exceeded 2.5 SDs below the sample mean (M = 8.08, cutoff = 6.5). Notably, this tSNR cutoff is nearly identical to the cutoff reported by Roalf and colleagues (Roalf et al. 2016) to optimally separate poor data from good quality data (6.47). There was no significant relationship between age and any of the motion metrics. There was a main effect of sex for percentage of slices with signal dropout such that females had a greater percentage than males, though both groups averaged less than one slice with signal dropout (males = 0.2%, females = 0.6%, t = −4.1, P < 0.001). There was a significant negative association between age and tSNR, which was driven by an age-by-sex interaction such that age was negative associated with tSNR in males but not females (age × sex parameter estimate = −0.06, SE = .018, P < 0.01; see Supplementary Fig. 1). Due to the significant association between age and tSNR, age regression models were performed with and without tSNR as a covariate. After eddy current and motion correction, the diffusion data were reconstructed and warped to standard space using q-space diffeomorphic reconstruction (Yeh and Tseng 2011) with a diffusion sampling length ratio of 1.25 using DSI Studio software (http://dsi-studio.labsolver.org). The output resolution was 2 mm.
Region of Interest Identification
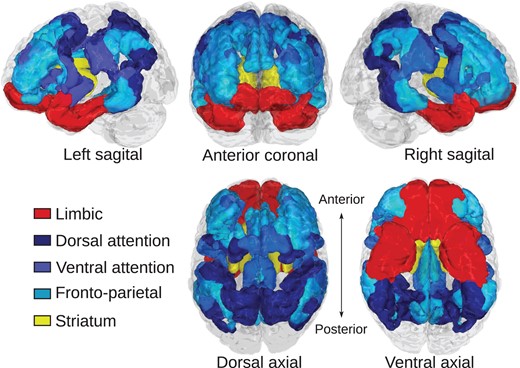
Cortical brain systems were identified according to the 7-network cortical atlas created by (Yeo et al. 2011) (Fig. 1). This atlas was chosen because it contained a cortical limbic system as well as cognitive control-related systems, and has been previously been used in studies of corticostriatal functional connectivity (Choi et al. 2012). We considered the fronto-parietal, dorsal attention, and ventral attention systems as systems related to aspects of cognitive control, and we considered the cortical limbic system to be involved reward processing and motivation (Fig. 1). The striatum was defined according to the Harvard-Oxford subcortical atlas distributed with FSL. Each region of interest (ROI) was split by left/right hemisphere. Gross identification and labeling of white matter fiber pathways was performed according to the “MRI Atlas of Human White Matter” (Mori et al. 2005).
Regions of interest for corticostriatal tractography. Cortical regions of interest relating to cognitive control function are colored in blue hues and include dorsal attention, ventral attention, and fronto-parietal systems. The cortical limbic system is colored in red. The striatum is colored yellow. Cortical regions were defined according to Yeo et al. (2011).
Deterministic Fiber Tracking
To identify corticostriatal pathways, we applied a deterministic fiber tracking algorithm (Yeh et al. 2013) to each participant’s reconstructed diffusion data using DSI Studio. Fiber tractography was performed for each cortical region of interest and the striatum, separately for each hemisphere. Whole-brain seeding was used and fiber streamlines were identified that passed through the cortical ROI and terminated (“END” mask in DSI Studio) in the striatum. The anisotropy threshold was set at 0.06. The angular threshold was 75°. The step size was 1 mm. The fiber trajectories were smoothed by averaging the propagation direction with 80% of the previous direction. A total of 5 million seeds were placed to ensure comprehensive detection of corticostriatal fibers across participants, and a connectivity map was calculated from the resulting tracts. We elected to use deterministic rather than probabilistic fiber tracking because our goal was to localize white matter targets to later quantify the connectivity value. Probabilistic fiber tracking provides a connectivity estimate that is solely based on computational simulation of the possible connections and a probability threshold is needed localize pathways. This connectivity definition is not necessarily related to axonal characteristics (Jbabdi and Johansen-Berg 2011) and interpretation of the results can be challenging. In our deterministic approach, connectivity is defined by the quantitative anisotropy (QA) value derived from diffusion MRI signals, which are more closely related to axonal characteristics. As such, deterministic fiber tracking is a better fit for this application.
Analyses
Convergent Zones
We first sought to determine the location of areas of the striatum that receive convergent corticostriatal inputs from limbic and cognitive control-related brain systems. To determine striatal fiber streamline endpoint locations for each cortical ROI, fiber streamline endpoint counts for striatal voxels were smoothed with a 4 mm kernel and thresholded at 1% of total striatal endpoints for each participant in order to estimate a more conservative endpoint map. The resulting maps were then binarized to form fiber tract endpoint masks. Individual participant striatal convergent zones were calculated as the intersections of the limbic projection field mask with the fronto-parietal, dorsal attention, and ventral attention projection field masks (i.e., 3 total convergent zones; limbic/fronto-parietal, limbic/dorsal attention, and limbic/ventral attention). Voxel-wise convergence probability masks were then generated from the entire sample. To determine where the convergence probability exceeded chance levels, we performed spatial permutation tests, randomly permuting the voxel indices of the pathway endpoint masks for all participants and calculating a null convergence probability distribution for each striatal voxel. Convergent zones were determined as clusters of voxels where the observed convergence probability for the sample significantly exceeded the null distribution with an alpha of 0.05, false discovery rate (FDR) corrected. To determine if the size or shape of convergent zones differed as a function of age, we performed voxel-wise logistic regression on the thresholded, binarized convergence maps across participants to test whether the likelihood of convergence differed by age across striatal voxels. Voxel-wise tests were multiple comparison corrected at a FDR alpha of 0.05.
Convergence Ratio
The convergence ratio thus varies between −1 and 1 such that a positive value indicates greater relative weighting of limbic projections and a negative value indicates greater weighting toward cognitive control projections.
Convergence ratio maturation regression models
| Convergent zone . | Variable . | Coefficient . | SE . | t . | P . |
|---|---|---|---|---|---|
| Limbic/fronto-parietal | |||||
| Left | |||||
| Age | −0.0029 | 0.0006 | −4.82 | <0.00001**** | |
| Sex | 0.0111 | 0.0060 | 1.85 | 0.067 | |
| Motion | |||||
| Translation | 0.0115 | 0.0201 | 0.57 | 0.568 | |
| Rotation | −0.0279 | 0.0188 | −1.48 | 0.142 | |
| Slice | −0.0735 | 0.5003 | −0.15 | 0.884 | |
| Global QA | −0.0751 | 0.0428 | −1.75 | 0.083 | |
| Right | |||||
| Age | −0.0035 | 0.0005 | −6.93 | <0.00001**** | |
| Sex | 0.0100 | 0.0049 | 2.03 | 0.046+ | |
| Motion | |||||
| Translation | 0.0243 | 0.0165 | 1.47 | 0.144 | |
| Rotation | −0.0457 | 0.0153 | −2.99 | 0.004* | |
| Slice | −0.0132 | 0.4340 | −0.03 | 0.976 | |
| Global QA | −0.0372 | 0.0339 | −1.10 | 0.276 | |
| Limbic/Ventral Attention | |||||
| Left | |||||
| Age | −0.0041 | 0.0012 | −3.31 | 0.001** | |
| Sex | 0.0328 | 0.0123 | 2.67 | 0.009* | |
| Motion | |||||
| Translation | 0.0744 | 0.0385 | 1.93 | 0.057 | |
| Rotation | −0.1214 | 0.0385 | −3.15 | 0.002** | |
| Slice | 0.4232 | 1.0407 | 0.41 | 0.685 | |
| Global QA | −0.1426 | 0.0770 | −1.85 | 0.067 | |
| Right | |||||
| 1/Age | 0.3626 | 0.2385 | 1.52 | 0.132 | |
| Sex | −0.0119 | 0.0083 | −1.44 | 0.154 | |
| Motion | |||||
| Translation | 0.0014 | 0.0276 | 0.05 | 0.960 | |
| Rotation | 0.0357 | 0.0267 | 1.34 | 0.185 | |
| Slice | −0.1321 | 0.7251 | −0.18 | 0.856 | |
| Global QA | 0.0069 | 0.0524 | 0.13 | 0.895 | |
| Convergent zone . | Variable . | Coefficient . | SE . | t . | P . |
|---|---|---|---|---|---|
| Limbic/fronto-parietal | |||||
| Left | |||||
| Age | −0.0029 | 0.0006 | −4.82 | <0.00001**** | |
| Sex | 0.0111 | 0.0060 | 1.85 | 0.067 | |
| Motion | |||||
| Translation | 0.0115 | 0.0201 | 0.57 | 0.568 | |
| Rotation | −0.0279 | 0.0188 | −1.48 | 0.142 | |
| Slice | −0.0735 | 0.5003 | −0.15 | 0.884 | |
| Global QA | −0.0751 | 0.0428 | −1.75 | 0.083 | |
| Right | |||||
| Age | −0.0035 | 0.0005 | −6.93 | <0.00001**** | |
| Sex | 0.0100 | 0.0049 | 2.03 | 0.046+ | |
| Motion | |||||
| Translation | 0.0243 | 0.0165 | 1.47 | 0.144 | |
| Rotation | −0.0457 | 0.0153 | −2.99 | 0.004* | |
| Slice | −0.0132 | 0.4340 | −0.03 | 0.976 | |
| Global QA | −0.0372 | 0.0339 | −1.10 | 0.276 | |
| Limbic/Ventral Attention | |||||
| Left | |||||
| Age | −0.0041 | 0.0012 | −3.31 | 0.001** | |
| Sex | 0.0328 | 0.0123 | 2.67 | 0.009* | |
| Motion | |||||
| Translation | 0.0744 | 0.0385 | 1.93 | 0.057 | |
| Rotation | −0.1214 | 0.0385 | −3.15 | 0.002** | |
| Slice | 0.4232 | 1.0407 | 0.41 | 0.685 | |
| Global QA | −0.1426 | 0.0770 | −1.85 | 0.067 | |
| Right | |||||
| 1/Age | 0.3626 | 0.2385 | 1.52 | 0.132 | |
| Sex | −0.0119 | 0.0083 | −1.44 | 0.154 | |
| Motion | |||||
| Translation | 0.0014 | 0.0276 | 0.05 | 0.960 | |
| Rotation | 0.0357 | 0.0267 | 1.34 | 0.185 | |
| Slice | −0.1321 | 0.7251 | −0.18 | 0.856 | |
| Global QA | 0.0069 | 0.0524 | 0.13 | 0.895 | |
Note. Bold indicates significant after multiple comparison correction.
+P < 0.05; *P < 0.05, **P < 0.01, ****P < 0.0001 Bonferroni corrected.
Convergence ratio maturation regression models
| Convergent zone . | Variable . | Coefficient . | SE . | t . | P . |
|---|---|---|---|---|---|
| Limbic/fronto-parietal | |||||
| Left | |||||
| Age | −0.0029 | 0.0006 | −4.82 | <0.00001**** | |
| Sex | 0.0111 | 0.0060 | 1.85 | 0.067 | |
| Motion | |||||
| Translation | 0.0115 | 0.0201 | 0.57 | 0.568 | |
| Rotation | −0.0279 | 0.0188 | −1.48 | 0.142 | |
| Slice | −0.0735 | 0.5003 | −0.15 | 0.884 | |
| Global QA | −0.0751 | 0.0428 | −1.75 | 0.083 | |
| Right | |||||
| Age | −0.0035 | 0.0005 | −6.93 | <0.00001**** | |
| Sex | 0.0100 | 0.0049 | 2.03 | 0.046+ | |
| Motion | |||||
| Translation | 0.0243 | 0.0165 | 1.47 | 0.144 | |
| Rotation | −0.0457 | 0.0153 | −2.99 | 0.004* | |
| Slice | −0.0132 | 0.4340 | −0.03 | 0.976 | |
| Global QA | −0.0372 | 0.0339 | −1.10 | 0.276 | |
| Limbic/Ventral Attention | |||||
| Left | |||||
| Age | −0.0041 | 0.0012 | −3.31 | 0.001** | |
| Sex | 0.0328 | 0.0123 | 2.67 | 0.009* | |
| Motion | |||||
| Translation | 0.0744 | 0.0385 | 1.93 | 0.057 | |
| Rotation | −0.1214 | 0.0385 | −3.15 | 0.002** | |
| Slice | 0.4232 | 1.0407 | 0.41 | 0.685 | |
| Global QA | −0.1426 | 0.0770 | −1.85 | 0.067 | |
| Right | |||||
| 1/Age | 0.3626 | 0.2385 | 1.52 | 0.132 | |
| Sex | −0.0119 | 0.0083 | −1.44 | 0.154 | |
| Motion | |||||
| Translation | 0.0014 | 0.0276 | 0.05 | 0.960 | |
| Rotation | 0.0357 | 0.0267 | 1.34 | 0.185 | |
| Slice | −0.1321 | 0.7251 | −0.18 | 0.856 | |
| Global QA | 0.0069 | 0.0524 | 0.13 | 0.895 | |
| Convergent zone . | Variable . | Coefficient . | SE . | t . | P . |
|---|---|---|---|---|---|
| Limbic/fronto-parietal | |||||
| Left | |||||
| Age | −0.0029 | 0.0006 | −4.82 | <0.00001**** | |
| Sex | 0.0111 | 0.0060 | 1.85 | 0.067 | |
| Motion | |||||
| Translation | 0.0115 | 0.0201 | 0.57 | 0.568 | |
| Rotation | −0.0279 | 0.0188 | −1.48 | 0.142 | |
| Slice | −0.0735 | 0.5003 | −0.15 | 0.884 | |
| Global QA | −0.0751 | 0.0428 | −1.75 | 0.083 | |
| Right | |||||
| Age | −0.0035 | 0.0005 | −6.93 | <0.00001**** | |
| Sex | 0.0100 | 0.0049 | 2.03 | 0.046+ | |
| Motion | |||||
| Translation | 0.0243 | 0.0165 | 1.47 | 0.144 | |
| Rotation | −0.0457 | 0.0153 | −2.99 | 0.004* | |
| Slice | −0.0132 | 0.4340 | −0.03 | 0.976 | |
| Global QA | −0.0372 | 0.0339 | −1.10 | 0.276 | |
| Limbic/Ventral Attention | |||||
| Left | |||||
| Age | −0.0041 | 0.0012 | −3.31 | 0.001** | |
| Sex | 0.0328 | 0.0123 | 2.67 | 0.009* | |
| Motion | |||||
| Translation | 0.0744 | 0.0385 | 1.93 | 0.057 | |
| Rotation | −0.1214 | 0.0385 | −3.15 | 0.002** | |
| Slice | 0.4232 | 1.0407 | 0.41 | 0.685 | |
| Global QA | −0.1426 | 0.0770 | −1.85 | 0.067 | |
| Right | |||||
| 1/Age | 0.3626 | 0.2385 | 1.52 | 0.132 | |
| Sex | −0.0119 | 0.0083 | −1.44 | 0.154 | |
| Motion | |||||
| Translation | 0.0014 | 0.0276 | 0.05 | 0.960 | |
| Rotation | 0.0357 | 0.0267 | 1.34 | 0.185 | |
| Slice | −0.1321 | 0.7251 | −0.18 | 0.856 | |
| Global QA | 0.0069 | 0.0524 | 0.13 | 0.895 | |
Note. Bold indicates significant after multiple comparison correction.
+P < 0.05; *P < 0.05, **P < 0.01, ****P < 0.0001 Bonferroni corrected.
Quantitative Anisotropy
We assessed fiber integrity with QA because this measure has been shown to be more robust to the influence of crossing fibers, which are common along corticostriatal tracts, and partial volume effects on estimates of diffusion anisotropy along the principle fiber direction than other indices, such as FA (Yeh et al. 2013; Zhang et al. 2013; Lim et al. 2015; Shen et al. 2015). This is because QA is calculated from a spin distribution function, estimated with q-space diffeomorphic reconstruction, that allows for the modeling of diffusion along multiple vectors. Diffusion modeled along fiber orientations that are inconsistent with the primary fiber orientation (e.g., crossing or branching fibers) do not bias the calculation of QA along the primary fiber direction (Yeh et al. 2013). Other indices, such as FA, can paradoxically increase overall when the anisotropy of an individual off-axis fiber population decreases (Pierpaoli et al. 2001). Further, our QA-based fiber tracking approach was evaluated in the 2015 ISMRM tractography challenge (http://www.tractometer.org/ismrm_2015_challenge/) as ID#03. The valid connection of the QA-based fiber tracking achieved the highest valid connections score (93%) among all 96 methods evaluated. In this study, we further improved the tracking results using a region of interest, and thus we could expect that the percentage valid connections should be greater than 90%. Although we still cannot assert that our tracking results are 100% accurate, the quality achieved should be among the best considering the state-of-the-art approach.
Regression Analyses
All regression analyses were performed using MATLAB 2016a (The Mathworks, Inc.). To determine age-related differences in the relative weighting of limbic and cognitive control-related convergent connections we regressed the convergence ratio on age and sex, covarying our 3 motion metrics and whole-brain (global) averaged QA using simple linear regression. To determine age-related differences in QA for tracks that influence the convergence ratio, we regressed QA on age and sex, covarying motion and global QA, separately for each tract. For all regression analyses, functional forms of age that have been previously shown to characterize age-related change during this period (Luna et al. 2004)—linear, inverse (1/age), and quadratic—were separately tested and model selection was performed among these functional forms using AIC. Cook’s distance was used to detect influence outliers based on the default MATLAB threshold of greater than 3 times the mean cook’s distance of the sample. Multiple comparison correction of p-values was done using the Bonferroni correction. Age-by-sex interactions were further included in the initial regression models. In the case of nonsignificant interaction effects, results were reported from regression models that included main effects only. The age variable was centered when interaction terms were included in the model.
Behavioral Assessment
As part of the original study protocol, participants performed an incentivized antisaccade task, which is used to assess incentive-modulated inhibitory control behavior. These data were collected in a separate visit that occurred 1–77 days (median = 17.5) prior to the diffusion MRI scan. The design of the task has been described in detail elsewhere (Paulsen et al. 2014). Here we report on the initial cross-sectional sample of the longitudinal sample reported on previously (Paulsen et al. 2014). We used these data for a follow-up analysis assessing the relationship between affective/cognitive control convergence ratios and incentive-modulated inhibitory control (which relies on both affective and cognitive control processes). In the task, participants received a cue indicating whether correct performance on the upcoming trial would result in an increase in points (reward trials), the prevention of a loss of points (loss trials), or not influence point totals (neutral trials). Participants were informed that their point total and the end of the experiment would lead to a monetary reward of up to US$25. Participants selected whether they would prefer this reward to be in the form of cash or a gift card of their choosing. Participants also indicated their subjective valuation of the US$25 reward using a 7-point Likert scale. All participants were provided this additional compensation at the end of the study. Following the incentive cue, a fixation-cross appeared on the computer screen for 1.5 s followed by a yellow dot that flashed in the periphery. To perform the trial correctly, participants had to make a saccade (monitored with eye tracking) to the side of the screen opposite the stimulus. The influence of reward incentives on inhibitory control performance was calculated as the difference in accuracy (commission errors only) for reward and neutral trials (loss trials were not included in analyses for this study). For behavioral analyses, this difference was regressed on age, controlling for sex. For brain-behavior analyses, this difference was regressed on the convergence ratio and age, covarying sex, motion metrics and global QA. For convergent zones in which the convergence ratio was significantly related to both age and behavior, we tested for mediation (i.e., convergence ratio as a mediator of age-related differences in behavior). Mediation models were statistically evaluated using bias-corrected bootstrap significance values over 5000 bootstraps and were implemented using the M3 Mediation Toolbox (https://canlabweb.CO.edu/wiki/doku.php/help/mediation/m3_mediation_fmri_toolbox; e.g., Wager et al. 2008). For all regression and mediation models including behavioral data, participants were excluded that were missing eye tracking data for greater than one-third of total trials in either condition (N = 8) or that performed at ceiling for both conditions (N = 14).
Results
Convergence of Corticostriatal Pathways
Convergent corticostriatal projections were identified between the cortical limbic system and the fronto-parietal and ventral attention systems in the rostral striatum but not the dorsal attention system (Fig. 2).
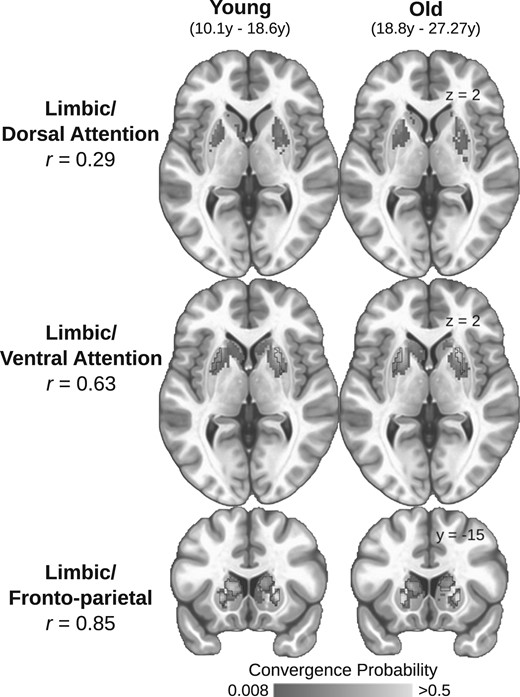
Spatially consistent convergent zones in adolescents and adults. The sample was split at the median age (18.6 years) and the striatal convergence probabilities for each pair of cortical ROIs were calculated for each group. The convergence probability maps between young (left column) and old (right column) participants and spatial correlations are presented for all convergence pairs (Top: limbic/dorsal attention r = 0.29; Middle: limbic/ventral attention r = 0.63; Bottom: limbic/fronto-parietal r = 0.85). Black outlines indicate the identified locations of the convergent zones used to conduct all subsequent analyses (as estimated from the entire sample). We did not observe a significant convergent zone for the limbic and dorsal attention systems.
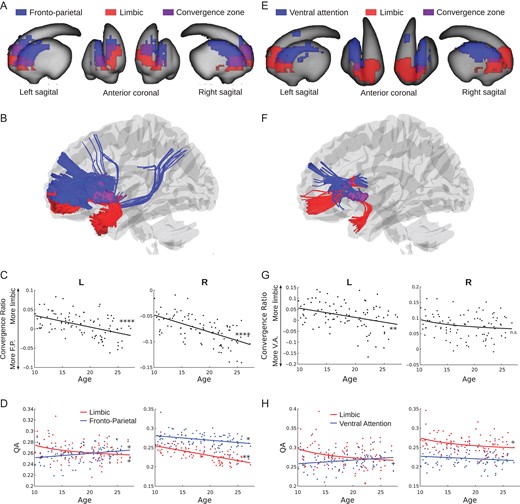
Zones of convergent corticostriatal projections between the fronto-parietal system, supporting on-line aspects of cognitive control (Dosenbach et al. 2007), and the cortical limbic system, supporting affective processes (reward and socioemotional processing), were observed bilaterally in clusters that extend from the head of the caudate to the anterior putamen (peak coordinates: −22,18,0 and 22,18,0 for left and right hemispheres, respectively) (Fig. 3A). The convergent zones encompassed 46.5% and 56% of the estimated limbic and fronto-parietal corticostriatal projection areas, respectively (Fig. 3A). These areas largely overlapped with areas of the striatum previously identified in a functional connectivity parcellation of the striatum to have primary functional connections with the limbic and fronto-parietal systems (Choi et al. 2012, Supplementary Fig. 2A). White matter tracts connecting each convergent zone to the fronto-parietal system included anterior aspects of the inferior and superior fronto-occipital fasciculi and posterior aspects of the superior fronto-occipital fasciculus. The inferior fronto-occipital and uncinate fasciculi connected each convergent zone to the cortical limbic system (Fig. 3B).
Affective/cognitive convergence assessed using quantitative anisotropy. (A) Limbic (red) and fronto-parietal (blue) corticostriatal fiber tracking striatal endpoints overlaid on the surface of the striatum. The convergent zone is colored in purple. (B) Fiber tracts connecting the limbic (red) and fronto-parietal (blue) cortical regions of interest to the striatal convergent zone from (A). (C) The convergence ratio significantly decreased with age throughout adolescence in both hemispheres (Table 1). (D) The individual maturational trajectories of limbic and fronto-parietal projections to the convergent zone. (E) Limbic (red) and ventral attention (blue) corticostriatal fiber tracking striatal endpoints overlaid on the surface of the striatum. The convergent zone is colored in purple. (F) Fiber tracts connecting the limbic (red) and ventral attention (blue) cortical regions of interest to the striatal convergent zone from (E). (G) The convergence bias significantly decreased with age throughout adolescence in the right hemisphere only. (H) The individual maturational trajectories of limbic and ventral attention projections to the convergent zone. + P < 0.05 uncorrected; *P < 0.05, **P < 0.01, ****P < 0.0001 Bonferroni corrected.
Corticostriatal projections from the ventral attention system, supporting sustained aspects of cognitive control and salience-based attention (Dosenbach et al. 2006, 2007), and limbic systems converged bilaterally in the anterior putamen (peak coordinates: −26,10,0 and 26,12,2 for left and right hemispheres, respectively) (Fig. 3E). The convergent zones encompassed 10% and 6% of the limbic and ventral attention corticostriatal projection areas, respectively (Fig. 3E). These areas largely overlapped with or were nearby to areas of the striatum previously identified in a functional connectivity parcellation of the striatum to have primary functional connections with the ventral attention and limbic systems (Choi et al. 2012, Supplementary Fig. 2B). White matter tracts connecting these convergent zones to the ventral attention system originated in the insular and middle frontal cortices. The inferior fronto-occipital and uncinate fasciculi connected each convergent zone to the cortical limbic system (Fig. 3F).
We did not detect a convergent zone for the dorsal attention system and the cortical limbic system. Though converging projections were detected in a small number of subjects, the probability of convergence did not exceed 7% for our sample (Fig. 2A). As such, subsequent analyses focus on limbic/fronto-parietal and limbic/ventral attention convergent zones.
Maturation of Convergent Corticostriatal Inputs
We first sought to examine whether the location or shape of the convergent zones differs with age. Voxel-wise logistic regression models indicated no striatal voxels significantly differed in the probability of being a convergent endpoint for any of the affective/cognitive convergence pairs as a function of age. This suggests that the spatial extent of the convergent zones does not expand or contract as a function of age and suggests the macro-level circuit architecture is in place by this stage of development. To further illustrate this, we split our sample at the median age (18.6 years), independently calculated convergence probabilities for both groups, and calculated the spatial correlation of convergence probabilities across striatal voxels. The probability maps between groups were highly correlated for the limbic/fronto-parietal (r = 0.85) and limbic/ventral attention (r = 0.63) convergent zones (Fig. 2), further indicating that convergent zones are spatially consistent across age-groups. Notably, despite the overall low observed convergence probability in the limbic/dorsal attention convergent zone, there was still a spatial correlation between old and young groups (r = 0.29).
We next quantified the relative integrity of the converging corticostriatal pathways that link affective and cognitive control-related cortical systems to the identified striatal convergent zones, the “convergence ratio” (see Materials and Methods), and examined its association with age in our sample. The limbic/fronto-parietal convergence ratio linearly decreases in both hemispheres throughout adolescence (Fig. 3C, Table 2), indicating that the relative weighting of limbic projections decreases throughout adolescence and into adulthood. This developmental change towards greater relative fronto-parietal weighting appears to be driven by a trend-level inverse linear age-related decrease in limbic QA in the left hemisphere (1/age coefficient = 0.27, SE = 0.12, P < 0.05 uncorrected) while fronto-parietal QA remained stable (coefficient = 0.006, SE = 0.004, n.s.) (Fig. 3D, left panel) and a greater age-related decrease in limbic QA (coefficient = −0.003, SE = 0.0003, P < 0.01 corrected) than fronto-parietal QA (coefficient = −0.001, SE = 0.0004, P < 0.05 Bonferroni corrected) (Fig. 3D, right panel). These developmental effects were not meaningfully changed by including tSNR as a covariate (Supplementary Table 1).
The limbic/ventral attention convergence ratio also linearly decreases with age, though the effect is only significant in the left hemisphere (Fig. 3G, Table 2). The age-related decrease in the left hemisphere convergence ratio appeared to be driven by a trend-level inverse linear age-related decrease in limbic QA (1/age coefficient = 0.45, SE = 0.2, P < 0.05 uncorrected) while ventral attention QA remained stable (coefficient = −0.0009, SE = 0.0005, n.s.) (Fig. 3H, left panel). The inclusion of tSNR as a covariate reduced the significance of the left hemisphere age coefficient to a trend-level effect, however tSNR itself was not a significant predictor of the convergence ratio (Supplementary Table 1), ruling out tSNR as a mediator of the relationship between age and the convergence ratio.
Sex Differences in Convergence Ratios
There was a significant main effect of sex in the left limbic/ventral attention convergent zone and a trend-level effect (P < 0.05 uncorrected) in the right limbic/fronto-parietal convergent zone (see Table 2). In both cases, the direction of the effect was such that males had greater (i.e., more limbic) limbic/cognitive control convergence ratios than females. There were no significant age-by-sex interactions.
Convergence Ratio and Incentive-Modulated Inhibitory Control
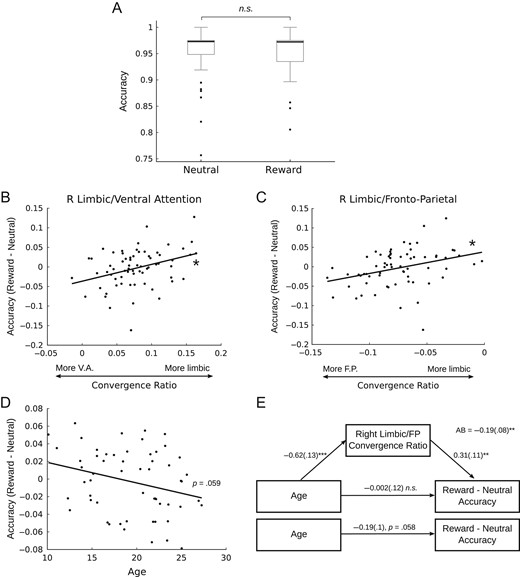
To determine whether the observed age-related differences in the affective/cognitive control convergence ratios were related to reward-related cognitive control performance, we performed follow-up analyses investigating the relationship between the convergence ratio and performance on an incentive-modulated inhibitory control task, the rewarded antisaccade. Although reward incentives did not significantly improve accuracy across the entire sample (Fig. 4A), we found that the right limbic/fronto-parietal and right limbic/ventral attention convergence ratios were positively associated with accuracy improvements under reward incentives (i.e., greater relative limbic weighting is associated with greater performance improvement) (Fig. 4B,C). Considering that accuracy was high overall in both conditions (Fig. 4A), as a follow-up analysis we compared the mean convergence ratios for only the participants who had the greatest difference in performance between conditions to test the hypothesis that those who have the greatest reward-related improvement in accuracy should have a greater (i.e., more limbic) convergence ratio. We found that participants who had a greater than 5% increase in accuracy in the “reward” condition (i.e., reward accuracy – neutral accuracy >5%; N = 5) had significantly more limbic convergence ratios than those who had a greater than 5% increase in performance in the “neutral” condition (i.e., reward accuracy – neutral accuracy <−5%; N = 8) in the bilateral limbic/fronto-parietal (Left: t = 2.21, P = 0.0495; Right: t = 2.82, P = 0.0155) and left limbic/ventral attention convergent zone (Left: t = 2.94, P = 0.0136; Right: t = 1.47, P = 0.176). Previous work using a rewarded antisaccade task (Padmanabhan et al. 2011) has demonstrated that the influence of reward incentives is greatest early in adolescence and diminishes into adulthood. Our findings generally support this pattern, though the effect was only significant at the trend level in this sample (Fig. 4D). Importantly, this effect not likely to be related to age-related differences in the subjective valuation of the reward incentive because we did not observe a significant association between age and the subjective valuations provided by participants (r = −0.05, P = 0.57). As the right limbic/fronto-parietal convergence ratio is significantly associated with both age (Fig. 3C) and performance (Fig. 4B), we sought to determine whether this effect mediated the trend-level association between age and reward-related performance improvements. Mediation analysis indicated that the right limbic/fronto-parietal convergence ratio significantly mediates the association between age and reward-related antisaccade performance (Fig. 4E).
The influence of the convergence ratio on reward-driven behavior. Participants completed an antisaccade task as a measure of inhibitory control. In one condition, participants were cued that they would be rewarded for correct performance (reward), in the other there was no reward contingency (neutral). There was no difference in overall accuracy between conditions (A), however there was a significant positive association between the right limbic/ventral attention (B) and right limbic/fronto-parietal (C) convergent zones such that a more limbic convergence ratio was associated with greater improvement to performance with reward incentives. (D) Improvements to accuracy with reward incentives trended toward being greater earlier in adolescence and diminishing into adulthood (P = 0.059). (E) The right limbic/fronto-parietal convergence ratio significantly mediated the trend-level association between age and incentive-based improvements in inhibitory control performance.
Discussion
Conceptual models of the neural basis for adolescent heightened reward drive, sensation seeking, and risk-taking suggest a developmental imbalance in the integration of affective and cognitive control systems and their resulting influence on behavior (Shulman et al. 2016); however, direct evidence for developmental differences in the integration of these systems has been lacking. We addressed this by showing how age-related differences in corticostriatal circuitry that integrates information from cortical limbic and control systems correlates with developmental differences in reward-driven behavior. Converging corticostriatal pathways form an infrastructure by which information from functionally distinct cortical systems can be integrated to influence action selection (Haber 2003, 2014; Averbeck et al. 2014; Verstynen 2014). Different cortical systems are selective for different feature representations and sensitive to different task contexts, stimuli, or goal states, in effect prioritizing different types of information (e.g., Klink et al. 2014). Whether competitive or complementary, this information must be integrated to select appropriate actions. This is accomplished, in part, by corticostriatal projections that are the inputs to the cortico-basal ganglia-thalamo-cortical pathways that bias action selection either directly (Humphries et al. 2006; Houk et al. 2007; Kimchi and Laubach 2009) or indirectly via action value representations (Frank 2005; Seo et al. 2012). In this way, striatal convergent zones function as one substrate for the integration of information from distinct cortical systems to influence behavior.
The influence of cortical systems on behavior should then vary with the relative connectivity integrity white matter projections to striatal convergent zones. To this end, we found that the relative integrity of convergent cortical affective (limbic) and cognitive control-related (fronto-parietal and ventral attention) pathways into the striatum differs with age, such that the relative integrity of affective pathways reduces throughout adolescence and into adulthood, coinciding with developmental changes in reward-guided decision making. Thus, these findings not only provide support for the notion that limbic systems have greater relative influence on behavior during adolescence than during adulthood (Shulman et al. 2016), they also provide one neurologically plausible mechanism by which these systems can influence behavior. Indeed, the right hemisphere limbic/fronto-parietal and limbic/ventral attention convergence ratios were positively associated with reward-related improvements in inhibitory control performance (Fig. 4).
The striatal convergent zones for the limbic/fronto-parietal and limbic/ventral attention systems identified were observed in the rostral aspects of the dorsal and ventral striatum. The spatial location of these convergent zones fell on or near the borders between areas of the striatum previously identified to be predominantly connected to the limbic and fronto-parietal or ventral attention regions investigated in this study (Supplementary Fig. 2), providing a notable consistency between functional (resting-state) and structural (dMRI) indices of corticostriatal connectivity. The cortical limbic system forms a cognitive map that prioritizes inferred economic or hedonic value via the OFC (Kringelbach 2005; Stalnaker et al. 2015), as well as socioemotional information via the temporal pole (Olson et al. 2007). Cortical cognitive control-related systems, on the other hand, prioritize goal-directed control of behavior. The fronto-parietal system is involved in transient aspects of cognitive control, such as control initiation, task-switching, and rule updating (Dosenbach et al. 2007; Cole et al. 2013). The ventral attention system prioritizes context-dependent visuospatial and perceptual salience, playing a functional role in orienting attention (Dosenbach et al. 2006; Fox et al. 2006; Fair et al. 2009). Considering these functional characteristics and the role of cortico-basal ganglia circuitry in influencing action selection (Seo et al. 2012; Wiecki and Frank 2013; Jin et al. 2014; Dunovan and Verstynen 2016), greater relative weighting of convergent cortical limbic projections in relation to projections from these cognitive control systems should bias adolescents to select actions or focus attention on items in the environment that have high inferred reward value even if those items are irrelevant to the present goal-state. This bias may then underlie the behavioral phenotype of greater reward sensitivity, a propensity for reward-driven behavior, and inconsistent cognitive control, which are hallmarks of adolescent behavior (Somerville and Casey 2010; Luna et al. 2015). Accordingly, we found that the right hemisphere limbic/fronto-parietal convergence ratio mediated age-related reductions in the influence of reward on inhibitory control performance. As has been previously reported (Padmanabhan et al. 2011; Geier and Luna 2012), the known developmental limitations in antisaccade performance during adolescence can be overcome in the presence of reward incentives. As we report here, this effect does not seem to be a function of developmental differences in the subjective value of reward stimuli. Rather, our present results indicate that this developmental phenomenon may be associated with the affective/cognitive control convergence ratio, particularly at limbic/fronto-parietal striatal convergent zones (Fig. 4E). In this experimental paradigm, where there is synergy between the cognitive demands and reward information, developmental differences in the limbic/cognitive control convergence ratio (i.e., greater relative weighting of limbic projections to limbic/cognitive control convergent zones during early adolescence) may form a neural substrate for the greater facilitation of task performance during rewarded trials for adolescents over adults. In this way, a greater affective/cognitive control convergence ratio may have adaptive qualities in early adolescence, facilitating, in this case, adult-like inhibitory control ability with incentive motivation.
We did not observe a convergent zone for the limbic and dorsal attention systems. This suggests that convergence of affective corticostriatal projections with other functional systems is selective even within the cognitive domain. The absence of a corticostriatal convergent zone is likely due to anatomical and topographical characteristics of the dorsal attention and limbic cortical systems. The dorsal attention system includes premotor, posterior parietal, and visual association brain regions which are topographically distal to the cortical limbic system (which includes orbitofrontal cortex and the temporal pole; Fig. 1) (Yeo et al. 2011). As a result, it’s corticostriatal connections are predominant in the caudal putamen whereas corticostriatal connections from the limbic system are predominant in the rostroventral striatum (Choi et al. 2012). The dorsal attention system is functionally involved in goal-directed sustaining of attention and is thus associated with sustained aspects of cognitive control (Fox 1995; Dosenbach et al. 2007). This is in contrast to the more transient control functions of the fronto-parietal and, to some extent, ventral attention systems (Dosenbach et al. 2007; Cole et al. 2013; Vossel et al. 2014). Speculatively, limbic system convergence with the fronto-parietal and ventral attention systems but not the dorsal attention system may indicate greater ability for reward information to interact with cognitive control in a transient manner, biasing task-switching and orienting of attention toward reward stimuli or contexts. Further, considering the close interaction between the ventral and dorsal attention systems to respectively orient and sustain attention (Vossel et al. 2014), there may not be a functional imperative for convergence between the limbic and dorsal attention system as the ventral attention system could function as an intermediary. Future work using functional imaging may help to delineate these complex functional interactions.
The development of the affective/cognitive control convergence ratio was predominantly driven by age-related decreases in the mean QA of cortical limbic projections to convergent zones while cognitive control projections generally remained stable, supporting the notion that systems supporting limbic function may be particularly influential in adolescence (Luna et al. 2015). Though developmental decreases in mean QA may appear surprising in consideration of studies assessing diffusion with the tensor model and reporting developmental increases in FA, it is important to note that these same studies typically also report developmental decreases in diffusion along the parallel axis, axial diffusivity, during adolescence (Qiu et al. 2008; Kumar et al. 2012; Simmonds et al. 2014), which in principle may be a more similar, though less robust (see Quantitative Anisotropy), measure to QA. This suggests that decreases in QA may speculatively reflect a developmental refinement in limbic corticostriatal structural connectivity. Notably, a recent longitudinal study similarly found age-related reductions in the QA of limbic (fronto-amygdalar) white patter pathways during adolescence (Jalbrzikowski et al. 2017). Additionally, a recent study (Baker et al. 2015) found decreased FA of subcortical tracts during late adolescence, which suggests developmental specialization may continue within subcortical systems throughout adolescence. These findings agree with resting-state functional connectivity MRI studies that find decreased fronto-striatal functional connectivity with age during adolescence (Supekar et al. 2009; Dosenbach et al. 2010; Padmanabhan et al. 2013; Porter et al. 2015). Considering that myelination may increase or decrease based on neuronal activity (Hines et al. 2015; Mensch et al. 2015), decreased functional connectivity and decreased white matter integrity observed here may be mechanistically interrelated.
We observed some evidence of hemispheric differences in our developmental and brain-behavior analyses. The right hemisphere limbic/ventral attention convergence ratio was not significantly associated with age in our sample, though the direction of the effect was consistent with the left hemisphere. We also observed that both the left hemisphere limbic/fronto-parietal and limbic/ventral attention did not have a significant linear association with incentive-modulated inhibitory control performance. However, when we focused our analyses only on participants with the greatest change in performance between conditions, we found that the left hemisphere convergence ratios did differentiate groups such that participants with greatest improvement in performance under reward incentives had a more limbic convergence ratio than those who had the greatest improvement in performance in the neutral condition, suggesting the left hemisphere convergent zones are still behaviorally relevant.
Sex differences impact many aspects of neural function and anatomy (Cahill and Aswad 2015) including white matter development (Wang et al. 2012; Simmonds et al. 2014). Here we find that males tended to have a greater limbic/cognitive control convergence ratio than females, with no significant age-by-sex interaction. This pattern of results suggests a greater influence of the cortical limbic system on male behavior relative to that of females regardless of age. This finding is in-line with recent work showing that adolescent males are more sensation seeking and have less impulse control than adolescent females (Shulman et al. 2015), and may play a role in sex differences in the development of striatum-related psychopathologies such as ADHD (Willcutt 2012) and substance abuse (Compton et al. 2007) which have greater incidence in males, and mood disorders, which have greater incidence in females (Cover et al. 2014).
Based on our specific hypotheses pertaining to developmental differences in the integration of affective and cognitive control systems and their influence on behavior throughout adolescence, our current study has selectively focused on the development of convergent corticostriatal projections from affective and cognitive control systems. We wish to acknowledge that convergent zones for other functional brain systems as well as corticostriatal projections from individual brain are also likely to play important functional roles in cognition and behavior and may also display important maturational changes throughout development. Future studies may further interrogate the development of these corticostriatal pathways to complement the findings of this study.
In summary, our findings indicate that early in adolescences the cortical affective system has the greatest relative integrity of projections into corticostriatal hubs that integrate affect and cognitive control information and that this neuroanatomical configuration is related to reward-driven behavior during this period of development. Thus, we propose that cortical projections to striatal convergent zones serve as one important developmental mechanism for the changing influence of affective and control systems on behavior, whereby the relative influence of affective systems decreases as adolescents make the transition to adulthood. Importantly, the greater influence of affective systems during early adolescence can be adaptive in nature in that it underlies an incentivized increase in cognitive control abilities. Developmental changes in the relative weighting of convergent corticostriatal projections may have implications that extend to abnormal development and behavior. Psychopathologies such as schizophrenia, substance abuse, and mood disorders emerge during adolescence and are associated with striatal abnormalities. As such, corticostriatal convergent zones may be a useful target for future clinical studies.
Supplementary Material
Supplementary data are available at Cerebral Cortex online.
Authors’ contributions
Conceptualization, Ba.L. and Be.L.; methodology, Ba.L., T.V., and F.Y.; software, Ba.L. and F.Y.; formal analysis, Ba.L.; writing—original draft, Ba.L.; writing—review and editing, Ba.L., Be.L., T.V., and F.Y.; supervision, Be.L. and T.V.; funding acquisition, Be.L.
Funding
This work was supported by the National Institutes of Health (grants R01 MH080243 and T90 DA022761).
Notes
Conflict of Interest: None declared.