-
PDF
- Split View
-
Views
-
Cite
Cite
Jeremiah N Jarrett, Specificity and costs of inducible defense in the barnacle Chthamalus fissus (Darwin, 1854), Journal of Crustacean Biology, Volume 38, Issue 5, September 2018, Pages 547–551, https://doi.org/10.1093/jcbiol/ruy052
Close - Share Icon Share
Abstract
Conditions under which plastic defenses of prey evolve include spatial and temporal variation in predator abundance, large-scale dispersal of prey propagules, a reliable predator cue and, costs associated with the defended morph in predator-free environments. The barnacle Chthamalus fissus Darwin, 1854 develops a narrow or bent aperture when exposed to the predatory snail Mexacanthina lugubris lugubris Sowerby, 1821. These “defended” individuals are better able to withstand predator assaults compared to the oval-aperture individuals. The study set out to determine: 1) whether induction of the narrow-aperture morph is a specific response to M. l. lugubris or a general response to snails that feed using a labral tooth, 2) if populations of C. fissus within the northern limit of M. l. lugubris have the same capacity to develop the narrow-aperture morph as the more southern populations and, 3) the costs associated with the two defended phenotypes. Mexacanthina l. lugubris, which ranges from Baja California, Mexico to Laguna Beach, CA, USA, and Acanthinucella spirata Blainville, 1832, which ranges from Washington state, USA to Baja California, both feed using a labral tooth. Chthamalus fissus populations from Las Olas, Baja California north to Laguna Beach, CA exhibit the same capacity to develop the narrow-aperture morph. Narrow-aperture individuals of C. fissus produced more eggs compared to oval- and bent-aperture individuals, which did not differ between themselves. Oval-aperture barnacles had significantly (P < 0.001) longer feeding limbs (cirri 4, 5, and 6) compared to narrow- and bent-aperture barnacles, which may improve feeding efficiency and explain the previously observed faster growth of oval-aperture individuals. It is suggested that narrow- and bent-aperture barnacles may begin reproducing at smaller sizes and that oval morphs of comparable size allocate energy to growth, postponing reproduction and achieving higher life-time fecundity than the defended morphs under predator-free conditions.
INTRODUCTION
Inducible defenses are an example of phenotypic plasticity in which individuals alter their behavior, morphology, or physiology in response to environmental threats, including predation (Murren et al., 2015). Predator-induced defense has been described for many taxonomic groups, (review by Harvell, 1990; Garay-Narvaez & Ramos-Jiliberto, 2009) and is most likely to evolve when: 1) prey species have a wide dispersal range, 2) predator populations are heterogeneously distributed in space and/or time, 3) predator-specific cues exist, and 4) fitness trade-offs differ among morphs in predator-free and predator-rich patches, such that canalized development of the defended morph would not be favored (Harvell, 1990). For marine invertebrates with wide dispersal abilities, the opportunity for genetic differentiation and character divergence among populations inhabiting different environments may be limited (Ehrlich & Raven, 1969; Slatkin, 1985; Sulton & Spencer, 2002), and under these conditions natural selection may favor the evolution of inducible defenses in response to predators (Wright, 1931; Bradshaw, 1965; Pigliucci, 2001; Hollander, 2008). The benefits of induced defense are balanced by the associated costs of defense, often described as nonconsumptive effects (NCE’s), which may include a reduced growth rate (Gosnell et al., 2017), reduced reproductive capacity (Lively, 1986b; van Buskirk & Steiner, 2009), and reduced competitive capacity (Tollrian & Harvell, 1999; Brönmark et al., 2012).
Inducible defenses vary depending on the nature of the threat experienced. Colonial bryozoans produce defensive stolons in response to competition for space on substrates, but they produce spines when exposed to nudibranch predators or high-flow conditions (Harvell, 1992). Prey species often display different induced defenses when exposed to predators that differ in attack mode (Harvell, 1992; DeWitt et al., 2000; Smith & Jennings, 2000; Bourdeau, 2009). The snail Nucella lamellosa (Gmelin, 1791) exhibits predator-specific plasticity, developing a rotund shell in the presence of a shell-crushing predator and an elongated shell with a narrow aperture in the presence of a shell-entry predator (Bourdeau, 2009). In southern New England, USA, blue mussels, Mytilus edulis (Linnaeus, 1758), develop thicker shells in response to green crabs, Carcinus maenas (Linneaus, 1758), and to the more recently introduced Asian shore crab, Hemigrapsus sanguineus (De Haan, 1835) (Freeman & Byers, 2006), suggesting that prey populations may evolve similar induced defenses in response to predators with similar attack modes. Induced defenses can result in selection in predators for improved predation capacity (Fordyce, 2006; Mougi et al., 2010), including the evolution of inducible offenses in predator populations (Kopp & Tollrian, 2003; Edgell & Rochette, 2009).
Geographic variation in the frequency of inducible genotypes within a prey population is likely a product of prey-dispersal capacity and asymmetry in the ranges and abundances of predator and prey populations (Lively et al., 2000; Trussell, 2000). For example, approximately 45% of individuals in populations of the barnacle Chthamalus anisopoma (Pilsbry, 1916) in the northern Gulf of California, developed a defensive phenotype (bent morph) when exposed to the gastropod predator Mexacanthina lugubris angelica (Oldroyd, 1918) while the remaining individuals developed the typical oval morphology regardless of the magnitude of predator cue experienced (Lively, 1986a; Lively et al., 2000). Lively (1999) speculated that the abundance of non-inducible genotypes of C. anisopoma in the northern Gulf of California was the result of larval transport from the southern gulf where the predator is absent and where, presumably, there is no selection favoring the inducible defense.
Introduced predators may also influence geographic variation in prey-inducible defenses. Populations of the blue mussel M. edulis in northern New England, where the Asian shore crab H. sanguineus is rare, are unable to develop thickened shells when exposed to this crab, whereas in southern New England, Asian shore crabs are abundant and induce blue mussels to thicken their shells (Freeman & Byers, 2006). Geographic variation in the frequency of inducible genotypes has an impact on the capacity of prey populations to persist as predator distributions change in response to global climate change (Sagarin et al., 1999; Walther et al., 2002; Parmesan et al., 2005) or as a result of anthropogenic introductions (Freeman & Byers, 2006) with variation in interaction strength between predator and prey potentially impacting community dynamics (Garay-Navaraz & Ramos-Jiliberto, 2009).
The barnacle C. fissus occurs along the southern Pacific coast of North America, with recent expansion northward (Barry et al., 1995). In addition to a standard oval-aperture morph, this species exhibits a defensive bent-aperture morph and a defensive narrow-aperture morph, both induced by exposure to the snail Mexacanthina lugubris lugubris Sowerby, 1821 (Jarrett, 2008, 2009). This occurrence of two alternative plastic defenses in response to the same predator appears to be unique among marine invertebrates. Although the bent-aperture barnacles are much less susceptible to predation, the narrow-aperture barnacles are the most common (85%) where M. l. lugubris is abundant, suggesting that the two morphs represent trade-offs in terms of costs of defense (Jarrett, 2008, 2009). Given that the cost associated with the predator-induced bent morph of C. anisopoma is reduced reproductive output and slower growth (Lively, 1986b), the narrow-aperture morph of C. fissus may persist because it is moderately resistant to predation and produced more eggs than bent-morph individuals of similar size. In addition to M. l. lugubris, the northern limit of which has expanded (Fenberg et al., 2014) as far north as Orange County, CA (Muhs et al., 2002), the predatory snail Acanthinucella spirata also overlaps in distribution with C. fissus and also feeds using a labral tooth.
Cypris larvae of Chthamalus fissus attach to and metamorphose on hard substrates in the upper intertidal zone, including the shells of molluscs (Newman & Abbott, 1980). This behavior allows for a natural field experiment in which one can compare the morphology of barnacles growing on shells of predatory and non-predatory gastropods that co-occur with this barnacle. An underlying assumption of this approach is that attached barnacles will be exposed to the chemical effluent of the host snail. In addition, many species of intertidal gastropods “huddle” in crevices when seeking refuge, and this would serve to increase the degree of exposure of attached barnacles to any chemical effluent.
The present study aimed to determine: 1) whether the induction of the defensive morph is a specific response to M. l. lugubris or a more generalized response to predatory snails that feed using a labral tooth, 2) whether the occurrence and degree of narrowing differs among northern and southern populations of C. fissus, and 3) the costs, if any, associated with the two defensive morphs.
MATERIALS AND METHODS
Specificity of inducible defense
Twelve to 20 live individuals each of two predatory snails, M. l. lugubris and A. spirata, and of two herbivorous snails, Lottia gigantea (Gray in G. B. Sowerby, 1834) and Tegula funebralis (A. Adams, 1855), mollusks commonly found within the C. fissus zone, were randomly collected in La Jolla, CA, USA (32o52ʹ12ʹʹN; 117o15ʹ15ʹʹW). Individuals of C. fissus (N = 25–90 per shell type) were identified, after preservation in 95% ethanol, by examining setae on cirrus 2 for a basal guard (Miller et al., 1989). Barnacle aperture lengths and widths were measured to the nearest 0.01mm using a dissecting microscope fitted with an ocular micrometer.
Geographic variation in defensive morphology
The degree of aperture narrowing was compared among the following three populations of C. fissus over a 200 km range, south to north: Las Olas, Baja California, Mexico (32°2ʹ49ʹʹN; 116°53ʹ11ʹʹW); La Jolla, CA, USA (32o52ʹ12ʹʹN; 117o15ʹ15ʹʹW); and Laguna Beach, CA, USA (33o29ʹ56ʹʹN; 117o44ʹ38ʹʹW). Living individuals of M. l. lugubris were randomly collected within the C. fissus zone at these three locations, and aperture lengths and widths of living, attached C. fissus barnacles were measured to the nearest 0.01mm.
Egg production
Approximately 300 C. fissus individuals were haphazardly collected in La Jolla, CA, USA (32o52ʹ1ʹʹN; 117o15ʹ15ʹʹW) within the zone occupied by adult individuals to compare size-specific egg production of the three morphs. The length and width of the aperture were measured and, if present, all eggs were removed and counted using a dissecting microscope. Both cirrus 1 were dissected and the length of each exopodite was measured to the nearest 0.01 mm using a compound microscope fitted with an ocular micrometer for use as a measure of adult size, since this mouth appendage has not been shown to vary with environmental cues in other barnacle species (Arsenault et al., 2001; Marchinko, 2003).
Length of feeding limbs
A potential cost associated with the defended morphs is reduced food capture efficiency which may result from shortened feeding limbs. Fifteen barnacles of each type morph were randomly collected in La Jolla, CA, USA (32o52ʹ12ʹʹN; 117o15ʹ15ʹʹW) within the zone occupied by adult C. fissus to determine if there was any difference in feeding leg length between morphs. The length and width of the aperture were measured for each individual after which the three feeding limbs (cirri 4, 5, and 6) from the left side of the prosoma were excised and the exopodite and endopodite were each measured from base to tip using a compound microscope equipped with an ocular micrometer (Arsenault et al., 2001). The average of the two measurements was used for analyses.
Statistical analysis
All data were analyzed using SPSSTM software (IBM SPSS Statistics for Macintosh Version 22.0). Data were transformed when necessary to meet the assumptions of ANCOVA. Geographic variation in C. fissus morphology on shells of M. l. lugubris was examined using ANCOVA with aperture width as the dependent variable, aperture length as a covariate, and site of origin as a fixed factor. Species specificity of the inducible response was examined using ANCOVA with the log of aperture width as the dependent variable, log of aperture length as a covariate, and shell type as a fixed factor. ANCOVA was used to test the null hypothesis that the log of egg production does not differ among the three morphs using morph as a fixed factor and cirrus 1 length as a covariate. When slopes were heterogeneous, the Wilcox (1987) modification of the technique of Johnson & Neyman (1936) (Quinn & Keough, 2002) was used to determine the range of covariates over which the treatments differed significantly from one another. The Wilcox (1987) modification of the original Johnson-Neyman technique allows for simultaneous determination of upper and lower limits of regions of non-significance for all pairs of treatments. The confidence level for all pairs of nonsignificance limits in the present study was set at 0.95.
RESULTS
Specificity of inducible defense
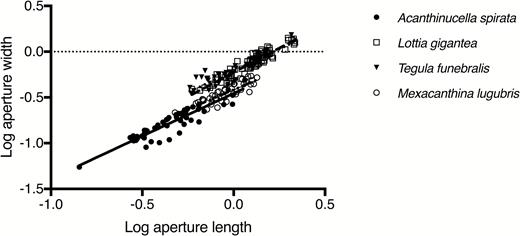
Aperture measurements (length and width) were log-transformed to meet the assumption of equal variances (Levene’s test, F = 2.11, P = 0.10). ANCOVA (Supplementary material Table S1) revealed that: 1) log aperture width increased with log aperture length (F = 856.5, P < 0.0001), 2) log aperture width varied among barnacles attached to the four different snail species (F = 128.0, P < 0.0001), and 3) the relationship between log aperture width and log aperture length of C. fissus differed among barnacles attached to the four shell types (F = 4.70, P = 0.003; Fig. 1). The Wilcox modification of the Johnson-Neyman technique showed that C. fissus growing on shells of Acanthinucella spirata were significantly narrower than those growing on M. l. lugubris up to an aperture length of 0.79 mm (back-transformed) and were significantly narrower than those growing on Lottia gigantea and Tegula funebralis over the entire range of sizes examined. Chthamalus fissus on M. l. lugubris were also significantly narrower than those on L. gigantea and T. funebralis over the range of sizes examined while barnacles growing on T. funebralis and L. gigantea did not differ in log aperture width over the range of sizes sampled (Fig. 1).
Log aperture width as a function of log aperture length for C. fissus on shells of: Aacnthina spirata, Mexacanthina lugubris lugubris, Tegula funebralis, and Lottia gigantea collected from La Jolla, CA, USA.
Geographic variation in defensive morphology
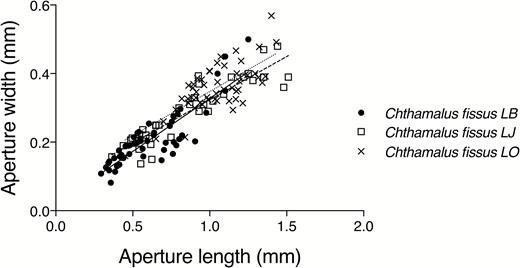
Aperture width measurements for C. fissus on shells of M. l. lugubris met the assumption for homogeneity of variances (Levene’s test, F = 2.3, P = 0.10). In general, aperture width increased with aperture length for barnacles growing on the shells of M. l. lugubris (ANCOVA, F = 301.3, P < 0.0001; Fig. 2) at the three study sites, and the relationship between aperture width and length for barnacles on shells of M. l. lugubris did not differ significantly (ANCOVA, test for interaction, F = 1.42, P = 0.25) among the three sites (Supplementary material Table S2).
Aperture width (mm) as a function of aperture length (mm) for barnacles Chthamalus fissus growing on Mexacanthina lugubris lugubris. LB, Laguna Beach, CA, USA; LJ, La Jolla, CA, USA; LO, Las Olas, Baja California, Mexico.
Egg production
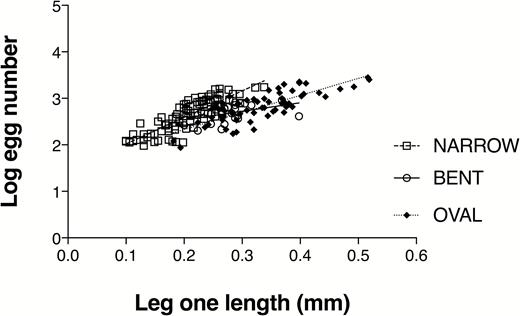
Egg counts were log-transformed to meet the assumption of equal variances (Levene’s test, F = 1.70, P = 0.186). Egg counts varied as a function of cirrus 1 exopodite length (ANCOVA, F = 111.9, P < 0.0001) and morph, (ANCOVA, F = 189.7, P < 0.0001) and the relationship between egg number and cirrus 1 exopodite length differed among all three morphs (ANCOVA, F = 11.8, P < 0.0001) (Supplementary material Table S3). The Wilcox modification of the Johnson-Neyman technique showed that narrow-aperture barnacles produced more eggs than oval- and bent-aperture barnacles of exopod lengths greater than 0.165 mm and 0.224 mm, respectively. Bent- and oval-aperture barnacles did not differ in egg production over the range of adult sizes examined (Fig. 3).
Log egg number as a function of cirrus 1 length (mm) for narrow-, oval-, and bent-aperture morphs of Chthamalus fissus from La Jolla, CA, USA.
Length of feeding limbs
Sample sizes were 10 or 11 due to the cirri of some individuals being damaged. Cirrus 6 measurements and aperture length measurements were log-transformed to meet the assumptions of homogeneity of variances (Levene’s Test, F = 1.49, P = 0.252) but the data for cirri 5 and 4 did not need to be transformed (Levene’s Test, F = 2.24, P = 0.125 and F = 1.93, P = 0.164, respectively). ANCOVA revealed that oval-aperture morphs had significantly longer feeding limbs than bent- and narrow-aperture morphs (P < 0.001 for all comparisons with oval morph cirrus length) and that bent- and narrow-aperture morphs only differed from each other in the length of cirrus 6 (P = 0.046), with that of the bent-aperture morph being the longer (Supplementary material Table S4).
DISCUSSION
The availability and reliability of predator cues is essential for the evolution of inducible prey defenses (Harvell, 1990; Lively, 1986c; Hazel et al., 2004; Bourdeau, 2010). Chthamalus fissus growing on shells of two different predators develop a narrow, defended morphology, suggesting that both predators release a reliable cue that C. fissus juveniles are able to detect and respond to. Furthermore, both predator species exhibit the same feeding strategy, using a labral tooth to penetrate the barnacle aperture. These results demonstrate that C. fissus exhibits the same inducible defense when exposed to predators sharing the same attack mode and that C. fissus aperture morphology does not change in response to non-predators. Chthamalus fissus growing on A. spirata were significantly narrower than those on M. l. lugubris up to an aperture length of 0.79 mm (back-transformed), suggesting that A. spirata induces more dramatic narrowing of the aperture of C. fissus. This could result from C. fissus being more sensitive to the A. spirata cue, or from A. spirata releasing more of the inducing cue than M. l. lugubris. The enhanced degree of narrowing by C. fissus could be advantageous given that A. spirata is a smaller snail with a relatively small labral tooth, but variation in the feeding success of A. spirata as a function of barnacle aperture narrowness was not investigated.
Barnacles growing on shells of M. l. lugubris at the three study sites showed the same degree of aperture narrowing. Mexacanthina l. lugubris is a relative newcomer to the intertidal communities in La Jolla and Laguna Beach, CA, USA (Muhs et al., 2002) whereas C. fissus and A. spirata have co-occurred in these communities for much longer. It is possible that defensive plasticity in C. fissus populations north of the historic northern limit of M. l. lugubris has been maintained in response to predation by A. spirata.
The greater egg production of narrow-aperture barnacles compared to bent-aperture barnacles of C. fissus indicates that, although narrow-aperture individuals are more susceptible to predation by M. l. lugubris (Jarrett, 2009), they also produce more larvae per reproductive event than bent-aperture barnacles of the same size. These data support previous findings of reproductive costs associated with induced defense in Chthamalus spp. (Lively, 1986b) and, more importantly, suggest that at least two alternative plastic genotypes may be maintained at the population or metapopulation level. Inducible defense in C. fissus is a successful strategy because individuals exposed to a predator have the opportunity to develop the defensive morphology before they reach the minimum size at which they are consumed by the predator (Lively et al., 2000). On a continuum of reproductive output and defense, narrow-aperture individuals may produce more eggs per reproductive event over a shorter life span compared to bent-aperture barnacles which maximize defense and, therefore, may live longer, but produce fewer offspring per reproductive event. Wide larval dispersal and geographic variation in the intensity of predation may serve to maintain both plastic genotypes along the southern Pacific coast of North America.
An unexpected finding was that oval-aperture barnacles generally produced fewer eggs than narrow-aperture barnacles and did not differ from bent-aperture barnacles in egg production. Juvenile oval-aperture barnacles, however, grow nearly twice as fast as juvenile narrow-aperture barnacles (Jarrett, 2009), which suggests that oval-aperture barnacles may allocate more energy to growth and competing for space and thus may defer reproduction to a larger size. The significantly longer feeding limbs of oval-aperture barnacles may explain their faster growth compared to narrow- and bent-aperture barnacles. Under predator-free conditions, it is predicted that oval-aperture barnacles would outcompete the narrow- and bent-aperture barnacles for space and thus have a higher lifetime reproductive success. Although C. fissus produces broods year-round (Page, 1984), we do not know whether the three morphs differ in the number of broods they produce annually or in age at first reproduction. Controlled predator-exposure experiments would allow for direct comparison of age and/or size at first reproduction, reproductive output, and reproductive frequency among the three morphs.
At locations where predatory snails are found, narrow-aperture barnacles are generally in greater abundance than are bent-aperture barnacles (Jarrett, 2008). It is possible that narrow-aperture individuals experience much lower non-consumptive effects and only slightly higher consumptive effects (Agrawal, 2001) compared to the bent-aperture individuals which would explain the higher reproductive output of narrow-aperture barnacles.
The results demonstrate that C. fissus responds similarly to two predators that share the same attack mode and suggest that two alternative plastic genotypes can co-occur in a population due to differences in reproductive output, vulnerability to predators, and temporal and spatial variation in predator abundance. The distribution and frequency of defensive genotypes in populations of coastal marine organisms is yet another factor that should be considered when attempting to manage coastal communities and predict community level responses to climate change and shifts in species ranges (Richards et al., 2010).
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of Crustacean Biology online.
S1 Table. ANCOVA for log aperture width of Chthamalus fissus.
S2 Table. ANCOVA for aperture width of Chthamalus fissus.
S3 Table. ANCOVA for log egg number of Chthamalus fissus.
S4 Table. ANCOVA for comparisons among the lengths of cirrus 6, 5, and 4 of the three morphs of Chthamalus fissus.
ACKNOWLEDGEMENTS
This project was supported by NSF Grant OCE-0083976 and Central Connecticut State University research grants. Thanks to F. Tapia, C.C. Jarrett, and C.F. Jarrett for field assistance and many BIO 390 and BIO 421 students at CCSU who happily measured snails and barnacles. This manuscript benefited greatly from reviews by anonymous reviewers, C.A. Penniman, and N. Dean. Thanks also to J. Pineda for inviting me to explore Biocomplexity.