-
PDF
- Split View
-
Views
-
Cite
Cite
Ian F. Pollack, Regina I. Jakacki, Lisa H. Butterfield, Ronald L. Hamilton, Ashok Panigrahy, Daniel P. Normolle, Angela K. Connelly, Sharon Dibridge, Gary Mason, Theresa L. Whiteside, Hideho Okada, Immune responses and outcome after vaccination with glioma-associated antigen peptides and poly-ICLC in a pilot study for pediatric recurrent low-grade gliomas, Neuro-Oncology, Volume 18, Issue 8, August 2016, Pages 1157–1168, https://doi.org/10.1093/neuonc/now026
Close - Share Icon Share
Abstract
Low-grade gliomas (LGGs) are the most common brain tumors of childhood. Although surgical resection is curative for well-circumscribed superficial lesions, tumors that are infiltrative or arise from deep structures are therapeutically challenging, and new treatment approaches are needed. Having identified a panel of glioma-associated antigens (GAAs) overexpressed in these tumors, we initiated a pilot trial of vaccinations with peptides for GAA epitopes in human leukocyte antigen–A2+ children with recurrent LGG that had progressed after at least 2 prior regimens.
Peptide epitopes for 3 GAAs (EphA2, IL-13Rα2, and survivin) were emulsified in Montanide-ISA-51 and administered subcutaneously adjacent to intramuscular injections of polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose every 3 weeks for 8 courses, followed by booster vaccines every 6 weeks. Primary endpoints were safety and T-lymphocyte responses against GAA epitopes. Treatment response was evaluated clinically and by MRI.
Fourteen children were enrolled. Other than grade 3 urticaria in one child, no regimen-limiting toxicity was encountered. Vaccination induced immunoreactivity to at least one vaccine-targeted GAA in all 12 evaluable patients: to IL-13Rα2 in 3, EphA2 in 11, and survivin in 3. One child with a metastatic LGG had asymptomatic pseudoprogression noted 6 weeks after starting vaccination, followed by dramatic disease regression with >75% shrinkage of primary tumor and regression of metastatic disease, persisting >57 months. Three other children had sustained partial responses, lasting >10, >31, and >45 months, and one had a transient response.
GAA peptide vaccination in children with recurrent LGGs is generally well tolerated, with preliminary evidence of immunological and clinical activity.
Low-grade gliomas (LGGs) are the most common primary brain tumors of childhood.1 Although they encompass several histologically discrete tumor types, the majority are pilocytic astrocytomas.1–3 Whereas surgical resection is curative for most superficial lesions of the cerebral and cerebellar hemispheres, the sizable group of LGGs that arise from deep structures, such as the diencephalon, are infrequently curable with conventional therapies and pose a major management challenge.2,3 Lesions that are progressive and/or unresectable often respond transiently to chemotherapy agents, such as carboplatin and vinblastine.2,4–9 Therefore, these approaches are often only temporizing strategies, and tumors frequently recur, often repetitively, leading to slowly progressing symptoms and chronic disability, particularly in lesions that fail multiple chemotherapy regimens and require irradiation.10 A subset become refractory to both chemotherapy and irradiation and are ultimately fatal.
One new approach for these tumors involves small-molecule–based strategies. Recent studies have demonstrated a high frequency of genomic alterations involving the mitogen activated protein kinase (MAPK) pathway in pilocytic astrocytomas, including BRAF-KIAA translocations, activating mutations of BRAF, and inactivating mutations of NF1, all of which constitutively activate MAPK signaling.11,12 Ongoing studies are examining the efficacy of MAPK pathway inhibitors13,14 for these tumors, although experience has shown that solid tumors can circumvent target-directed inhibitors by acquiring new mutations or signaling alterations that confer resistance.15,16 Thus, notwithstanding the significant promise of such approaches, it is unlikely that they will be individually curative, and additional treatment modalities will almost certainly be warranted.
Cancer vaccines are promising in this regard, designed to induce systemic immunity against antigens overexpressed by tumor cells. Pilot clinical trials by us17,18 and others19–23 have demonstrated the safety and potential efficacy of peripheral vaccinations for adults with malignant gliomas. Vaccine approaches may be even more effective if applied early in treatment and in clinical contexts where patients are likely to have intact immunity, such as during childhood or young adulthood.24–27
The current trial was based upon the use of peptide epitopes for 3 glioma-associated antigens (GAAs) that we observed to be highly expressed in pediatric gliomas: IL-13Rα2, EphA2, and survivin.28 The human leukocyte antigen (HLA)–A2-restricted cytotoxic T-lymphocyte epitopes included 2 that we had previously identified, an interleukin-13 receptor (IL-13R)α2 analog peptide (IL-13Rα2345-353:1A9V)29,30 and EphA2883-891,31 as well as survivin96-104:M2,32,33 admixed with a pan-HLA-DR tetanus toxoid peptide (TetA830-845), administered in a mineral oil base (Montanide), which has been shown in murine models to induce high levels of antigen-specific cytotoxic T-lymphocytes,34 eliminating the need for dendritic cell harvesting. The vaccine was delivered in conjunction with the immunoadjuvant polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose (poly-ICLC), which has been shown to enhance the efficacy of tumor antigen vaccination in glioma-bearing mice34,35 and to be safely administered to adults with malignant glioma,17,36–38 and in conjunction with vaccination in our pilot trial for children with high-grade and brainstem gliomas.27
The current study was designed to evaluate vaccination with GAA epitopes in combination with poly-ICLC in pediatric patients with recurrent LGGs. We hypothesized that this regimen would prove to be safe and would induce potent antiglioma immune responses.
Patients and Methods
Patients
Patients between 1 and 21 years of age with recurrent LGGs were eligible. These included children with biopsy-confirmed LGG or nonbiopsied optic pathway glioma who had neurofibromatosis 1, with disease that was unresectable and progressive after at least 2 prior treatment regimens. Adequate liver, renal, and other organ function, Karnofsky or Lansky performance status ≥60, and HLA-A2+ status were required, and patients must have recovered from the effects of prior therapies, being at least 3 weeks from the last dose of cytotoxic chemotherapy or myelosuppressive biological therapy and at least 1 week from the last dose of nonmyelosuppressive biologic therapy. The maximal allowable dexamethasone dose during the week before beginning vaccination was 0.1 mg/kg/d (maximum 4 mg/d). The trial was conducted under FDA Investigator New Drug application No. 13624 and institutional review board protocol PRO08030085 and was registered with ClinicalTrials.gov (No. NCT01130077). Signed informed consent was required for both HLA screening and initiation of therapy.
Study Design
Patients received subcutaneous injections of GAA-derived HLA-A*0201–restricted peptides and a pan-HLA-DR tetanus toxoid peptide (TetA830-845) emulsified in Montanide-ISA-51 (Seppic) in 800 µL, and adjacent intramuscular injections of the toll-like receptor ligand, poly-ICLC (30 µg/kg Hiltonol; Oncovir), administered on an outpatient basis every 3 weeks (defined as a course) for a total of 8 vaccines. Participants were evaluated for adverse events, regimen-limiting toxicity (RLT), and treatment response by clinic visits, laboratory testing, and MRI. Immune response was assessed at baseline and 6, 15, and 21 weeks after start of vaccination by enzyme-linked immunosorbent spot (ELISPOT) assay on peripheral blood mononuclear cells (PBMCs), timed to coincide with MRI scans. Patients demonstrating radiological response (ie, complete or partial response [PR]) or stable disease (SD) without RLT from the vaccine had the option of continuing vaccination at 6-week intervals for up to 2 years from the initial vaccine, with immunological and MRI evaluations at 12-week intervals, coinciding with every other visit for vaccine administration.
Toxicity Assessment and Stopping Rules
The trial was monitored continuously for treatment-related adverse events using National Cancer Institute Common Toxicity Criteria version 3.0. The following were considered to be RLTs if they were judged possibly, probably, or definitely associated with treatment: grade ≥2 hypersensitivity or allergic reaction; grade ≥3 nonhematologic toxicity; grade ≥3 hematologic toxicity that recurred despite 33% poly-ICLC dose reduction or did not resolve by the time the next dose was due. A stopping rule for excessive toxicity was defined as an observed rate of RLT ≥33%, if at least 2 RLTs had been observed.
Peptides
HLA-A2–restricted peptides used in this trial were: ALPFGFILV (IL-13Rα2345-353:1A9V),30 TLADFDPRV (EphA2883-891),31 LMLGEFLKL (survivin96-104:M2),32,33 admixed with a pan-DR helper epitope AQYIKANSKFIGITEL (TetA830-845).39 The peptides were synthesized by automated solid-phase peptide synthesis by NeoMPS (PolyPeptide Group). Peptides were tested in multiple quality-assurance studies including purity, sterility, identity, potency, pyrogenicity, and stability.
ELISPOT Assays
ELISPOT assays were performed on PBMCs obtained and cryopreserved before vaccination (wk 0), and at weeks 6, 15, 21, and every (q)12 weeks. Batched PBMC samples from each patient were evaluated simultaneously following 7-day in vitro stimulation with IL-13Rα345-353, EphA2883-891, and survivin96-104 peptides, 10 ng/mL IL-7, and 20 IU/mL IL-2. Interferon (IFN)-γ responses by purified CD8+ and CD4+ T cells were tested against T2 cells pulsed with GAA peptides or PBMCs pulsed with TetA830-845, respectively. A positive ELISPOT response was defined as >2-fold increase in net spot-forming T cells (after background subtraction) (CD8+ cells for GAAs, CD4+ cells for TetA830-845) over the pre-vaccine level and at least 50 spots/100 000 cells.
Radiological Response Monitoring
Tumor size was assessed before vaccination and at weeks 6, 15, 21, and q12 weeks thereafter using MRI scans with contrast enhancement. More frequent scans were obtained if clinically warranted as described below. Response was evaluated by gadolinium-enhanced T1-weighted images, T2-weighted images, or both, based upon the appearance of the pretreatment MRI.
Management of Immunological Pseudoprogression
In our previous vaccine trials in adults17,40 and children27 with malignant gliomas, a subset of participants had symptomatic pseudoprogression, characterized by transient increased size of the tumor and/or increased contrast enhancement secondary to intratumoral immune response, followed by tumor stabilization or regression and symptom improvement.41 Accurately managing such patients is essential to avoid both premature termination of therapy and unacceptable neurological deterioration. Accordingly, the trial incorporated detailed management guidelines regarding dexamethasone administration and delaying subsequent vaccination, as recently described in detail in our pilot vaccine study for children with high-grade and brainstem gliomas.27 Children with asymptomatic pseudoprogression were permitted to continue on vaccination, provided tumor cross-sectional area was not increased by more than 25% above the pre-event baseline.
Statistical Methods
This pilot study was designed to enroll 12 evaluable children with recurrent LGGs to assess safety and immunological efficacy. The treatment approach was considered worthy of further investigation if there were at least 5 ELISPOT responses amongst 12 evaluable subjects. In addition, we planned to stop accrual if the rate of RLT was ≥33% and at least 2 RLTs were observed. Patients with disease progression during the first 2 courses of therapy were replaced for RLT analysis. Survival functions were estimated using the product-limit (Kaplan–Meier) method and compared by log-rank tests. Greenwood's method was used to calculate confidence regions for the survival function estimates. Cox proportional hazards regression was employed to relate progression-free survival (PFS) to changes in ELISPOT reactivity. Wilcoxon rank-sum tests were used to compare continuous variables across groups.
Results
Demographics and Clinical Characteristics
Between April 2011 and March 2015, forty-six children with recurrent LGGs were screened for HLA status; 17 (37%) were HLA-A2+, of whom 3 were not enrolled because they either opted for other studies (n = 2) or did not have evidence of MRI-confirmed disease progression. Fourteen children were enrolled (Table 1), including 1 with neurofibromatosis 1–related optic pathway tumor that had not undergone biopsy confirmation. Nine of the children had pilocytic astrocytomas, 2 had pilomyxoid astrocytomas, and 2 had desmoplastic infantile ganglioglioma. None of the patients was receiving dexamethasone at the time of study enrollment. Patients received 2–21 courses of therapy (median = 10). Twelve children completed at least 2 vaccine courses and were evaluable for immunological response; 1 each exhibited RLT (Patient 8) and disease progression (Patient 7) during their second courses and were not evaluable for immune response.
Demographics and clinical and immunological responses for each patient
| Patient ID . | Age at Start, y . | Gender . | Tumor Type . | Tumor Location . | Prior Therapy . | Maximal ELISPOT Responses . | Best Response . | Vaccines Received . | Systemic Toxicities . | PFS . | OS . | PTP . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-13Rα . | EphA2 . | Survivin . | Tet . | ||||||||||||
| 1 | 10.5 | M | PA | Hypothalamus | Carbo VBL | 36 | 250 | 42 | 2 | SD | 15 | Gr 1 bruising, fatigue, fever, flu-like symptoms; Gr 2 injection site reaction, induration, myalgias, nausea, rash | 16.1 | 42.0 | |
| 2 | 14.4 | F | PA | Hypothalamic/optic chiasm | TPCV VBL CC5013 Bev/Irin | 112 | 46 | 106 | 44 | SD | 19 | Gr 2 headache, injection site reaction induration | 20.8 | 57.3 | |
| 3 | 17.6 | F | PA | Hypothalamic/optic chiasm | Ifos/VP16 Carbo/VCR TMZ VBL Bev/Irin | 16 | 102 | 20 | 172 | SD | 8 | Gr 1 increased ALT/AST, anorexia, decreased, bruising, diarrhea, Gr 2 fatigue, fever, hypermagnesemia, injection site reaction, induration nausea, stomach pain, thigh pain, vomiting | 6.7 | 49.1 | |
| 4 | 10.9 | F | PA | Left temporal lobe | VBL Carbo TMZ Oxal Lenalid Bev/Irin | 194 | 216 | 60 | 8 | PR | 21 | Gr 1 anorexia, fatigue, fever, headache, hypermagnesemia, hypophosphatemia, Gr 2 injection site reaction, induration, leg pain, nausea, vomiting | 57.3 | 57.3 | Yes |
| 5 | 19.0 | M | PMA | Thalamus/basal ganglia | Carbo VBL TMZ | 96 | 382 | 20 | 4 | PR | 21 | Gr 1 body aches, fatigue, flu-like symptoms, headache, Gr 2 injection site reaction, induration, nausea | 45.7 | 45.7 | |
| 6 | 13.0 | F | OPT/NF | Optic chiasm | Carbo/VCR VBL | 6 | 182 | 4 | 10 | SD | 11 | Gr 1 body aches, Gr 2 injection site reaction, induration | 9.8 | 44.6 | |
| 71 | 6.6 | M | PMA | Hypothalamic/optic chiasm | Carbo/VCR VBL Evero Bev/Irin | NA | NA | NA | NA | PD | 2 | Gr 1 headache, induration | 2.7 | 41.9 | |
| 82 | 5.0 | M | DIG | Posterior 3rd ventricle | Carbo/VCR CPM/Cis/VCR/VP16 Bev VBL | NA | NA | NA | NA | Not evaluable | 2 | Gr 1 chills, vomiting, fatigue, Gr 2 fever, injection site reaction, induration, rash, Gr 3 hives | 1.1 | 42.2 | |
| 9 | 7.8 | F | DIG | Right temporal/visual pathway | Carbo CPM/Cis/VCR/VP16 | 12 | 62 | 14 | 0 | MR | 10 | Gr 2 fever, injection site reaction, induration | 9.9 | 41.0 | |
| 10 | 16.1 | M | PA | Hypothalamic/optic chiasm | Carbo/VCR TMZ Thalid/VP16//CPM VBL Lenalid | 44 | 58 | 20 | 20 | SD | 9 | Gr 1 chills, fever, flu-like symptoms, injection site reaction, induration | 8.0 | 40.5 | |
| 11 | 13.6 | F | PA | Hypothalamic/optic chiasm | Carbo TMZ | 20 | 276 | 104 | 44 | PR | 21 | Gr 1 anemia, bruising, headache, Gr 2 injection site reaction, induration | 31.2 | 31.2 | |
| 12 | 1.9 | M | PA | Cerebellum | Carbo/VCR Carbo/VBL | 28 | 300 | 2 | 12 | SD | 7 | Gr 1 vomiting, fatigue, fever, flu-like symptoms, injection site reaction, induration | 7.5 | 27.8 | |
| 13 | 7.5 | M | PA | Left frontal temporal lobe | TMZ Carbo | 14 | 1046 | 12 | 444 | SD | 5 | Gr 1 bruising, fatigue, injection site reaction, induration | 3.5 | 24.3 | |
| 14 | 9.8 | M | PA | Spinal cervical thoracic | Carbo VBL Bev | 42 | 492 | 44 | 0 | PR | 10 | Gr 1 bruising, fever, Gr 2 injection site reaction, induration | 10.7 | 10.7 | Yes |
| Patient ID . | Age at Start, y . | Gender . | Tumor Type . | Tumor Location . | Prior Therapy . | Maximal ELISPOT Responses . | Best Response . | Vaccines Received . | Systemic Toxicities . | PFS . | OS . | PTP . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-13Rα . | EphA2 . | Survivin . | Tet . | ||||||||||||
| 1 | 10.5 | M | PA | Hypothalamus | Carbo VBL | 36 | 250 | 42 | 2 | SD | 15 | Gr 1 bruising, fatigue, fever, flu-like symptoms; Gr 2 injection site reaction, induration, myalgias, nausea, rash | 16.1 | 42.0 | |
| 2 | 14.4 | F | PA | Hypothalamic/optic chiasm | TPCV VBL CC5013 Bev/Irin | 112 | 46 | 106 | 44 | SD | 19 | Gr 2 headache, injection site reaction induration | 20.8 | 57.3 | |
| 3 | 17.6 | F | PA | Hypothalamic/optic chiasm | Ifos/VP16 Carbo/VCR TMZ VBL Bev/Irin | 16 | 102 | 20 | 172 | SD | 8 | Gr 1 increased ALT/AST, anorexia, decreased, bruising, diarrhea, Gr 2 fatigue, fever, hypermagnesemia, injection site reaction, induration nausea, stomach pain, thigh pain, vomiting | 6.7 | 49.1 | |
| 4 | 10.9 | F | PA | Left temporal lobe | VBL Carbo TMZ Oxal Lenalid Bev/Irin | 194 | 216 | 60 | 8 | PR | 21 | Gr 1 anorexia, fatigue, fever, headache, hypermagnesemia, hypophosphatemia, Gr 2 injection site reaction, induration, leg pain, nausea, vomiting | 57.3 | 57.3 | Yes |
| 5 | 19.0 | M | PMA | Thalamus/basal ganglia | Carbo VBL TMZ | 96 | 382 | 20 | 4 | PR | 21 | Gr 1 body aches, fatigue, flu-like symptoms, headache, Gr 2 injection site reaction, induration, nausea | 45.7 | 45.7 | |
| 6 | 13.0 | F | OPT/NF | Optic chiasm | Carbo/VCR VBL | 6 | 182 | 4 | 10 | SD | 11 | Gr 1 body aches, Gr 2 injection site reaction, induration | 9.8 | 44.6 | |
| 71 | 6.6 | M | PMA | Hypothalamic/optic chiasm | Carbo/VCR VBL Evero Bev/Irin | NA | NA | NA | NA | PD | 2 | Gr 1 headache, induration | 2.7 | 41.9 | |
| 82 | 5.0 | M | DIG | Posterior 3rd ventricle | Carbo/VCR CPM/Cis/VCR/VP16 Bev VBL | NA | NA | NA | NA | Not evaluable | 2 | Gr 1 chills, vomiting, fatigue, Gr 2 fever, injection site reaction, induration, rash, Gr 3 hives | 1.1 | 42.2 | |
| 9 | 7.8 | F | DIG | Right temporal/visual pathway | Carbo CPM/Cis/VCR/VP16 | 12 | 62 | 14 | 0 | MR | 10 | Gr 2 fever, injection site reaction, induration | 9.9 | 41.0 | |
| 10 | 16.1 | M | PA | Hypothalamic/optic chiasm | Carbo/VCR TMZ Thalid/VP16//CPM VBL Lenalid | 44 | 58 | 20 | 20 | SD | 9 | Gr 1 chills, fever, flu-like symptoms, injection site reaction, induration | 8.0 | 40.5 | |
| 11 | 13.6 | F | PA | Hypothalamic/optic chiasm | Carbo TMZ | 20 | 276 | 104 | 44 | PR | 21 | Gr 1 anemia, bruising, headache, Gr 2 injection site reaction, induration | 31.2 | 31.2 | |
| 12 | 1.9 | M | PA | Cerebellum | Carbo/VCR Carbo/VBL | 28 | 300 | 2 | 12 | SD | 7 | Gr 1 vomiting, fatigue, fever, flu-like symptoms, injection site reaction, induration | 7.5 | 27.8 | |
| 13 | 7.5 | M | PA | Left frontal temporal lobe | TMZ Carbo | 14 | 1046 | 12 | 444 | SD | 5 | Gr 1 bruising, fatigue, injection site reaction, induration | 3.5 | 24.3 | |
| 14 | 9.8 | M | PA | Spinal cervical thoracic | Carbo VBL Bev | 42 | 492 | 44 | 0 | PR | 10 | Gr 1 bruising, fever, Gr 2 injection site reaction, induration | 10.7 | 10.7 | Yes |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; Bev, bevacizumab; Carbo, carboplatin; Cis, cisplatin; CPM, cyclophosphamide; DIG, desmoplastic infantile gangliogliomas; Evero, everolimus; Gr: grade; Ifos, ifosfamide; Irin, irinotecan; Lenalid, lenalidomide; MR, minor response; NF1, neurofibromatosis 1; OPT, optic pathway tumor (diagnosed based on MRI); OS, overall survival; Oxal, oxaloplatin; PA, pilocytic astrocytoma; PD, progressive disease; PMA, pilomyxoid astrocytoma; PTP, pseudo-tumor progression; PXA, pleomorphic xanthoastrocytoma; Tet, tetanus toxoid; TMZ, temozolomide; TPCV, 6-thioguanine, procarbazine, CCNU (lomustine), VCR; VBL, vinblastine; VCR, vincristine; VP16, etoposide.
Toxicities: highest toxicities are noted.
Results of maximal IFN-γ ELISPOT reactivity (net spots/105 cells after background subtraction) are shown for each epitope. Values ≥50 were considered positive and reflected at least a 2-fold increase compared with pre-vaccine. Positive responses are shown in bold. NA indicates samples that were not available because of progression or RLT before completion of the second vaccine course.
Patients who remain progression free are indicated in bold. 1Patient removed from study for early progression. 2Patient removed from the study for regimen-limiting grade 3 hives. For prior therapy, each treatment regimen is indicated by a separate line in the table.
Demographics and clinical and immunological responses for each patient
| Patient ID . | Age at Start, y . | Gender . | Tumor Type . | Tumor Location . | Prior Therapy . | Maximal ELISPOT Responses . | Best Response . | Vaccines Received . | Systemic Toxicities . | PFS . | OS . | PTP . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-13Rα . | EphA2 . | Survivin . | Tet . | ||||||||||||
| 1 | 10.5 | M | PA | Hypothalamus | Carbo VBL | 36 | 250 | 42 | 2 | SD | 15 | Gr 1 bruising, fatigue, fever, flu-like symptoms; Gr 2 injection site reaction, induration, myalgias, nausea, rash | 16.1 | 42.0 | |
| 2 | 14.4 | F | PA | Hypothalamic/optic chiasm | TPCV VBL CC5013 Bev/Irin | 112 | 46 | 106 | 44 | SD | 19 | Gr 2 headache, injection site reaction induration | 20.8 | 57.3 | |
| 3 | 17.6 | F | PA | Hypothalamic/optic chiasm | Ifos/VP16 Carbo/VCR TMZ VBL Bev/Irin | 16 | 102 | 20 | 172 | SD | 8 | Gr 1 increased ALT/AST, anorexia, decreased, bruising, diarrhea, Gr 2 fatigue, fever, hypermagnesemia, injection site reaction, induration nausea, stomach pain, thigh pain, vomiting | 6.7 | 49.1 | |
| 4 | 10.9 | F | PA | Left temporal lobe | VBL Carbo TMZ Oxal Lenalid Bev/Irin | 194 | 216 | 60 | 8 | PR | 21 | Gr 1 anorexia, fatigue, fever, headache, hypermagnesemia, hypophosphatemia, Gr 2 injection site reaction, induration, leg pain, nausea, vomiting | 57.3 | 57.3 | Yes |
| 5 | 19.0 | M | PMA | Thalamus/basal ganglia | Carbo VBL TMZ | 96 | 382 | 20 | 4 | PR | 21 | Gr 1 body aches, fatigue, flu-like symptoms, headache, Gr 2 injection site reaction, induration, nausea | 45.7 | 45.7 | |
| 6 | 13.0 | F | OPT/NF | Optic chiasm | Carbo/VCR VBL | 6 | 182 | 4 | 10 | SD | 11 | Gr 1 body aches, Gr 2 injection site reaction, induration | 9.8 | 44.6 | |
| 71 | 6.6 | M | PMA | Hypothalamic/optic chiasm | Carbo/VCR VBL Evero Bev/Irin | NA | NA | NA | NA | PD | 2 | Gr 1 headache, induration | 2.7 | 41.9 | |
| 82 | 5.0 | M | DIG | Posterior 3rd ventricle | Carbo/VCR CPM/Cis/VCR/VP16 Bev VBL | NA | NA | NA | NA | Not evaluable | 2 | Gr 1 chills, vomiting, fatigue, Gr 2 fever, injection site reaction, induration, rash, Gr 3 hives | 1.1 | 42.2 | |
| 9 | 7.8 | F | DIG | Right temporal/visual pathway | Carbo CPM/Cis/VCR/VP16 | 12 | 62 | 14 | 0 | MR | 10 | Gr 2 fever, injection site reaction, induration | 9.9 | 41.0 | |
| 10 | 16.1 | M | PA | Hypothalamic/optic chiasm | Carbo/VCR TMZ Thalid/VP16//CPM VBL Lenalid | 44 | 58 | 20 | 20 | SD | 9 | Gr 1 chills, fever, flu-like symptoms, injection site reaction, induration | 8.0 | 40.5 | |
| 11 | 13.6 | F | PA | Hypothalamic/optic chiasm | Carbo TMZ | 20 | 276 | 104 | 44 | PR | 21 | Gr 1 anemia, bruising, headache, Gr 2 injection site reaction, induration | 31.2 | 31.2 | |
| 12 | 1.9 | M | PA | Cerebellum | Carbo/VCR Carbo/VBL | 28 | 300 | 2 | 12 | SD | 7 | Gr 1 vomiting, fatigue, fever, flu-like symptoms, injection site reaction, induration | 7.5 | 27.8 | |
| 13 | 7.5 | M | PA | Left frontal temporal lobe | TMZ Carbo | 14 | 1046 | 12 | 444 | SD | 5 | Gr 1 bruising, fatigue, injection site reaction, induration | 3.5 | 24.3 | |
| 14 | 9.8 | M | PA | Spinal cervical thoracic | Carbo VBL Bev | 42 | 492 | 44 | 0 | PR | 10 | Gr 1 bruising, fever, Gr 2 injection site reaction, induration | 10.7 | 10.7 | Yes |
| Patient ID . | Age at Start, y . | Gender . | Tumor Type . | Tumor Location . | Prior Therapy . | Maximal ELISPOT Responses . | Best Response . | Vaccines Received . | Systemic Toxicities . | PFS . | OS . | PTP . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-13Rα . | EphA2 . | Survivin . | Tet . | ||||||||||||
| 1 | 10.5 | M | PA | Hypothalamus | Carbo VBL | 36 | 250 | 42 | 2 | SD | 15 | Gr 1 bruising, fatigue, fever, flu-like symptoms; Gr 2 injection site reaction, induration, myalgias, nausea, rash | 16.1 | 42.0 | |
| 2 | 14.4 | F | PA | Hypothalamic/optic chiasm | TPCV VBL CC5013 Bev/Irin | 112 | 46 | 106 | 44 | SD | 19 | Gr 2 headache, injection site reaction induration | 20.8 | 57.3 | |
| 3 | 17.6 | F | PA | Hypothalamic/optic chiasm | Ifos/VP16 Carbo/VCR TMZ VBL Bev/Irin | 16 | 102 | 20 | 172 | SD | 8 | Gr 1 increased ALT/AST, anorexia, decreased, bruising, diarrhea, Gr 2 fatigue, fever, hypermagnesemia, injection site reaction, induration nausea, stomach pain, thigh pain, vomiting | 6.7 | 49.1 | |
| 4 | 10.9 | F | PA | Left temporal lobe | VBL Carbo TMZ Oxal Lenalid Bev/Irin | 194 | 216 | 60 | 8 | PR | 21 | Gr 1 anorexia, fatigue, fever, headache, hypermagnesemia, hypophosphatemia, Gr 2 injection site reaction, induration, leg pain, nausea, vomiting | 57.3 | 57.3 | Yes |
| 5 | 19.0 | M | PMA | Thalamus/basal ganglia | Carbo VBL TMZ | 96 | 382 | 20 | 4 | PR | 21 | Gr 1 body aches, fatigue, flu-like symptoms, headache, Gr 2 injection site reaction, induration, nausea | 45.7 | 45.7 | |
| 6 | 13.0 | F | OPT/NF | Optic chiasm | Carbo/VCR VBL | 6 | 182 | 4 | 10 | SD | 11 | Gr 1 body aches, Gr 2 injection site reaction, induration | 9.8 | 44.6 | |
| 71 | 6.6 | M | PMA | Hypothalamic/optic chiasm | Carbo/VCR VBL Evero Bev/Irin | NA | NA | NA | NA | PD | 2 | Gr 1 headache, induration | 2.7 | 41.9 | |
| 82 | 5.0 | M | DIG | Posterior 3rd ventricle | Carbo/VCR CPM/Cis/VCR/VP16 Bev VBL | NA | NA | NA | NA | Not evaluable | 2 | Gr 1 chills, vomiting, fatigue, Gr 2 fever, injection site reaction, induration, rash, Gr 3 hives | 1.1 | 42.2 | |
| 9 | 7.8 | F | DIG | Right temporal/visual pathway | Carbo CPM/Cis/VCR/VP16 | 12 | 62 | 14 | 0 | MR | 10 | Gr 2 fever, injection site reaction, induration | 9.9 | 41.0 | |
| 10 | 16.1 | M | PA | Hypothalamic/optic chiasm | Carbo/VCR TMZ Thalid/VP16//CPM VBL Lenalid | 44 | 58 | 20 | 20 | SD | 9 | Gr 1 chills, fever, flu-like symptoms, injection site reaction, induration | 8.0 | 40.5 | |
| 11 | 13.6 | F | PA | Hypothalamic/optic chiasm | Carbo TMZ | 20 | 276 | 104 | 44 | PR | 21 | Gr 1 anemia, bruising, headache, Gr 2 injection site reaction, induration | 31.2 | 31.2 | |
| 12 | 1.9 | M | PA | Cerebellum | Carbo/VCR Carbo/VBL | 28 | 300 | 2 | 12 | SD | 7 | Gr 1 vomiting, fatigue, fever, flu-like symptoms, injection site reaction, induration | 7.5 | 27.8 | |
| 13 | 7.5 | M | PA | Left frontal temporal lobe | TMZ Carbo | 14 | 1046 | 12 | 444 | SD | 5 | Gr 1 bruising, fatigue, injection site reaction, induration | 3.5 | 24.3 | |
| 14 | 9.8 | M | PA | Spinal cervical thoracic | Carbo VBL Bev | 42 | 492 | 44 | 0 | PR | 10 | Gr 1 bruising, fever, Gr 2 injection site reaction, induration | 10.7 | 10.7 | Yes |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; Bev, bevacizumab; Carbo, carboplatin; Cis, cisplatin; CPM, cyclophosphamide; DIG, desmoplastic infantile gangliogliomas; Evero, everolimus; Gr: grade; Ifos, ifosfamide; Irin, irinotecan; Lenalid, lenalidomide; MR, minor response; NF1, neurofibromatosis 1; OPT, optic pathway tumor (diagnosed based on MRI); OS, overall survival; Oxal, oxaloplatin; PA, pilocytic astrocytoma; PD, progressive disease; PMA, pilomyxoid astrocytoma; PTP, pseudo-tumor progression; PXA, pleomorphic xanthoastrocytoma; Tet, tetanus toxoid; TMZ, temozolomide; TPCV, 6-thioguanine, procarbazine, CCNU (lomustine), VCR; VBL, vinblastine; VCR, vincristine; VP16, etoposide.
Toxicities: highest toxicities are noted.
Results of maximal IFN-γ ELISPOT reactivity (net spots/105 cells after background subtraction) are shown for each epitope. Values ≥50 were considered positive and reflected at least a 2-fold increase compared with pre-vaccine. Positive responses are shown in bold. NA indicates samples that were not available because of progression or RLT before completion of the second vaccine course.
Patients who remain progression free are indicated in bold. 1Patient removed from study for early progression. 2Patient removed from the study for regimen-limiting grade 3 hives. For prior therapy, each treatment regimen is indicated by a separate line in the table.
Summary of Toxicities
The primary objective of this study was to assess safety. Principal systemic toxicities, summarized in Table 1, included grades 1 and 2 injection site reactions in 14 children and flu-like symptoms (fatigue, myalgias, fever, chills, headache) in 13, the latter of which were usually limited to 24 to 48 h after each vaccine and were controlled with acetaminophen or ibuprofen. Grade 1 or 2 gastrointestinal toxicity (nausea, vomiting) was observed in 6 children. One child (Patient 8) had a grade 2 urticarial reaction after the first vaccine, which rapidly resolved but recurred with the second vaccine as a grade 3 reaction, despite lowering the poly-ICLC dose as per the protocol; this was considered an RLT, and therapy was discontinued. One other child (Patient 7) with a prior history of intratumoral hemorrhage developed another episode of intratumoral hemorrhage in association with disease progression after the second vaccine, which was considered unrelated to the vaccine, and treatment was discontinued. No grade 2 or higher hematologic toxicity or incidences of autoimmunity were encountered.
Pseudoprogression
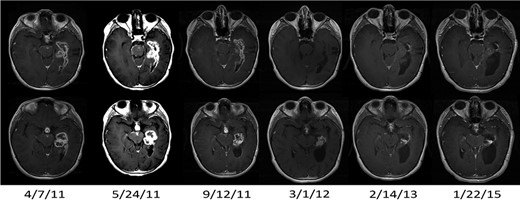
One child (Patient 4) with a progressive LGG had asymptomatic pseudoprogression after the second vaccine that did not necessitate interruption of protocol therapy, with transient enlargement in tumor size and enhancement followed by progressive and dramatic shrinkage of the primary tumor and resolution of subependymal and leptomeningeal metastatic disease persisting for >57 months, >33 months after conclusion of the 2-year vaccination period (Fig. 1). One other child (Patient 14) exhibited a PR but had symptomatic pseudoprogression manifested by neurological worsening with gait deterioration after the eighth vaccine and received dexamethasone per protocol (as well as bevacizumab by the treating neuro-oncologist, which was a protocol deviation). The patient improved symptomatically, bevacizumab was stopped, the decadron dose was weaned, and the patient resumed vaccine therapy and remains on study >10 months after beginning the vaccine regimen.
MRI results (T1-weighted gadolinium-enhanced axial images) in a responding patient (Patient 4) with transient pseudoprogression. Baseline scan date is 4/7/11. Pseudoprogression was apparent on the scan after 2 vaccines (5/24/11). Scans from subsequent dates show regression of leptomeningeal and subependymal enhancement and decreased size of the primary tumor, which has persisted over time. The PR status has been maintained for >57 mo after diagnosis.
Induction of Epitope-Specific Immune Responses against Glioma-Associated Antigens
All but 2 patients, who had disease progression and RLT, respectively, before completion of the second vaccine cycle, had PBMCs available for immunological analysis. One child, who is still on study, is having ongoing analyses but has data through week 21. In all 12 patients evaluated to date, vaccination induced immune reactivity to at least one of the vaccine-targeted GAA epitopes by IFN-γ ELISPOT assays (Table 1): to IL-13Rα2 in 3, EphA2 in 11, and survivin in 3.
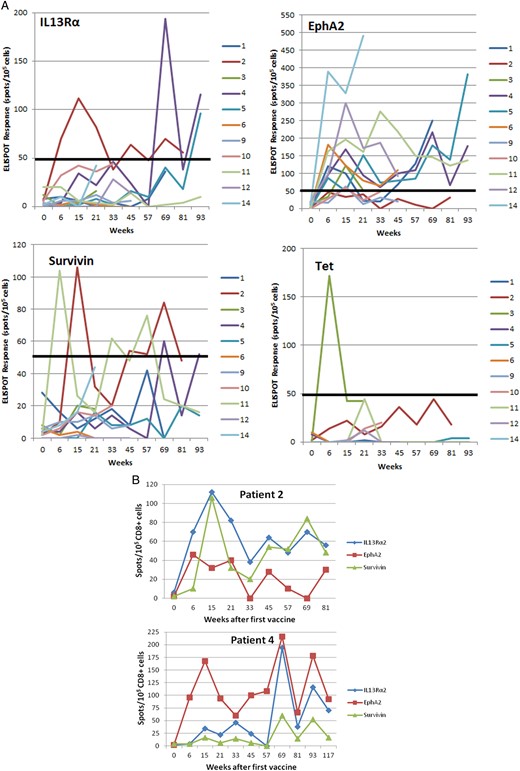
The time course and magnitude of the ELISPOT responses are summarized in Fig. 2A. The epitope that most consistently demonstrated the highest magnitude of response among the GAA peptides administered was EphA2. In responding patients, immunoreactivity generally persisted over extended intervals and, in Patients 4, 5, and 11, was observed to continue throughout the 2-year vaccine course (Fig. 2A and B).
(A) Time course of GAA epitope-specific T-cell responses evaluated by IFN-γ ELISPOT analyses in patients who had samples available at week 0 (pre-vaccine) and at weeks 6, 15, and 21. Points represent net values after background subtraction. A positive ELISPOT response was defined as >2-fold increase in net spot-forming T cells (after background subtraction) (CD8+ cells for GAAs, CD4+ cells for TetA830-845) over the pre-vaccine level and at least 50 spots/100 000 cells (indicated by a thick solid line). (B) ELISPOT responses in 2 patients. Upper: Patient 2, who had stable disease for >20 mo on vaccine therapy, demonstrated persistent ELISPOT responses to both IL-13Rα2 and survivin. Lower: Patient 4, whose MRI scans are depicted in Figure 1, demonstrated a significant partial response of the primary tumor and complete regression of all metastatic disease. Persistent positive ELISPOT responses to both IL-13Rα2 and EphA2 were observed throughout the course of vaccination and persisted after completion of the 2-year vaccine regimen.
Clinical Outcomes
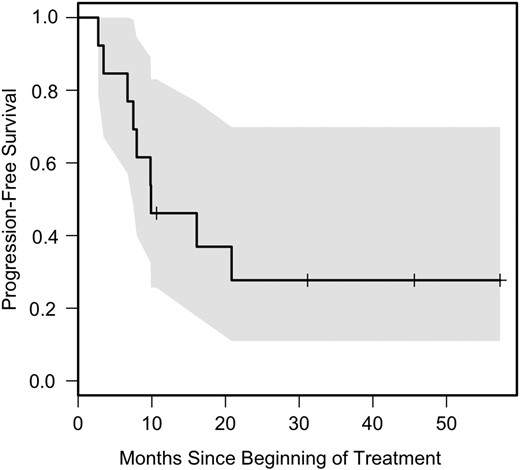
Although the goal of this study was to provide an analysis of safety and tolerability, preliminary efficacy data were obtained. Only one patient had disease progression during the initial 2 cycles of therapy, and one other had RLT before completion of the second course. Among the remaining patients, the best radiographic response was sustained PR (>50% tumor shrinkage) in Patients 4, 6, 11, and 14; minor response (25%–50% decrease in tumor size) that was transient in Patient 9; and SD in 7 patients. Thirteen LGG patients were evaluable for PFS and overall survival; one patient, noted above, was not evaluable due to RLT with the second vaccine without progression. The median PFS was 9.9 months (95% CI: 7.5 mo, not attained) (Fig. 3). Six-month PFS was achieved by 11/13 (85%) patients (95% CI: 55%–98%), while 5/12 patients (42%; 95% CI: 15%–72%) experienced 12-month PFS. Patient 14 remains alive at 10.7 months without progression and is therefore included in the 6-month but not 12-month calculations. Overall survival was 100%, with a median follow-up of 42 months. All 10 patients who were withdrawn from vaccine therapy for progression (9) or toxicity (1) received additional treatments, most commonly selumetinib (n = 6) and gamma knife irradiation (n = 2), and the overall survival results therefore reflect the effect of additional therapy.
PFS for 13 evaluable patients. The shaded area represents a 95% confidence region.
Clinical/Immunological Correlations
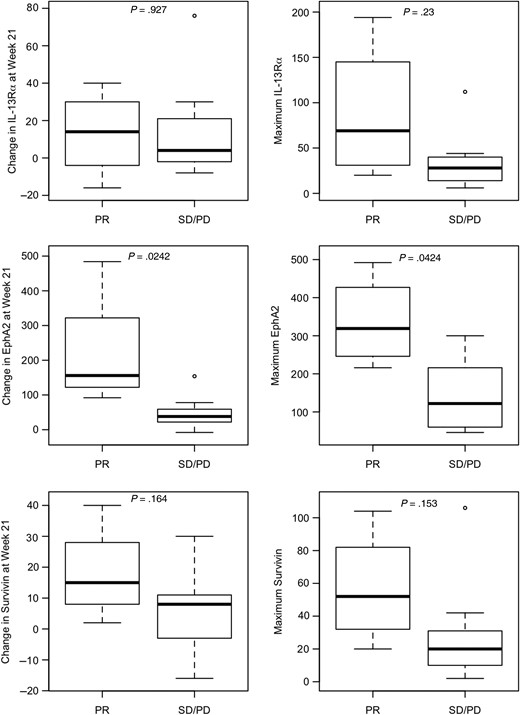
In the 11 patients who had samples available at week 0 (pre-vaccine) and at the week 6, 15, and 21 timepoints, increases in ELISPOT reactivity for EphA2 between baseline and week 21 and between baseline and maximum reactivity observed for EphA2 were related to clinical response (sustained PR vs minor response or SD with eventual progression, P = .024 and .042, respectively) (Fig. 4). Weaker trends were noted for the survivin epitope. Proportional hazards (Cox) regression was used to relate PFS to change in ELISPOT response to a given vaccine-targeted epitope from week 0 to week 21, and from week 0 to the maximum value obtained. Trends were observed between PFS and EphA2 response at week 21 and maximum change in IL-13Rα2 and survivin (all P = .06; Table 2). Patients who exhibited positive ELISPOT responses to 2 or more antigens (4/11) (Table 1) were more likely to have objective radiological responses than those who responded to only a single antigen (P = .09, Fisher's exact test). Because none of the patients underwent surgical resection during or after vaccine therapy, an assessment of immune response in the tumor itself was not possible.
Association between ELISPOT response and PFS
| Antigen Target . | Change from Baseline to Week 21 . | Change from Baseline to Maximum . |
|---|---|---|
| IL-13Rα2 | .88 | .06 |
| EphA2 | .06 | .09 |
| Survivin | .29 | .06 |
| Antigen Target . | Change from Baseline to Week 21 . | Change from Baseline to Maximum . |
|---|---|---|
| IL-13Rα2 | .88 | .06 |
| EphA2 | .06 | .09 |
| Survivin | .29 | .06 |
Proportional hazards (Cox) regression was used to relate PFS to change in ELISPOT response to a given vaccine-targeted epitope from week 0 to week 21, and from week 0 to the maximum value obtained. P-values for the tests are reported. A trend was noted between PFS and EphA2 response at week 21 as well as the maximum response to IL-13Rα2 and survivin.
Association between ELISPOT response and PFS
| Antigen Target . | Change from Baseline to Week 21 . | Change from Baseline to Maximum . |
|---|---|---|
| IL-13Rα2 | .88 | .06 |
| EphA2 | .06 | .09 |
| Survivin | .29 | .06 |
| Antigen Target . | Change from Baseline to Week 21 . | Change from Baseline to Maximum . |
|---|---|---|
| IL-13Rα2 | .88 | .06 |
| EphA2 | .06 | .09 |
| Survivin | .29 | .06 |
Proportional hazards (Cox) regression was used to relate PFS to change in ELISPOT response to a given vaccine-targeted epitope from week 0 to week 21, and from week 0 to the maximum value obtained. P-values for the tests are reported. A trend was noted between PFS and EphA2 response at week 21 as well as the maximum response to IL-13Rα2 and survivin.
Differences between patients with PR (n = 4) and minor response/SD with eventual progressive disease (PD; n = 7) in terms of the increase in ELISPOT reactivity for IL-13Rα2, EphA2, and survivin peptides between baseline and week 21 (left) and between baseline and maximal reactivity (right). P-values are derived from Wilcoxon's rank-sum test.
Discussion
This is the first clinical evaluation of peptide-based vaccination using novel GAA-derived epitopes in an emulsion-based vehicle, administered in conjunction with immunoadjuvant therapy for recurrent childhood LGGs. Our findings demonstrate reasonable safety and immunological efficacy of this approach, as well as preliminary evidence of clinical activity.
Although children with progressive LGG often exhibit transient disease control with a variety of chemotherapeutic regimens, such as carboplatin and vincristine,2,8 nitrosourea-based chemotherapy,2,6 vinblastine,4,42 temozolomide,7 and lenalidomide,9 the majority of patients eventually exhibit disease progression. Conformally targeted irradiation43 has shown efficacy in achieving disease control but raises concerns about late morbidity, particularly in young patients and those with extensive disease. Recent molecular studies have identified a high frequency of genomic alterations involving the MAPK pathway in pediatric low-grade astrocytomas,11,12 which constitutes a promising therapeutic target,13 although only a subset of tumors respond to MAPK pathway inhibition and some of the responders eventually experience toxicity.14 Accordingly, novel treatment strategies, such as immunotherapy, warrant consideration as additional therapeutic options.
The peptide epitopes included in this vaccine were derived from 3 proteins known to be highly expressed in pediatric gliomas.28 ELISPOT data demonstrated that all evaluable vaccinated patients mounted an immune response against at least one of the target antigens, supporting the use of such epitopes in pediatric glioma vaccine regimens. In contrast to our results in newly diagnosed high-grade or brainstem gliomas, where loss of target antigen reactivity over time was a common phenomenon,27 ELISPOT reactivity in the LGG cohort was generally maintained throughout the time of vaccine administration. The basis for tumor progression in many of the patients with ELISPOT responses therefore remains conjectural but may reflect issues of immune escape, involving outgrowth of tumor subclones not expressing targeted antigens44 or lacking antigen processing machinery components, such as major histocompatibility complex antigens,45,46 or the development of an unfavorable immune milieu mediated by regulatory T-cell populations47 or upregulation of immune checkpoint molecules,48 issues that warrant evaluation in future studies.
Although our response rate and PFS results for patients with recurrent LGG suggest an encouraging degree of activity, it is important to emphasize that this was a pilot study focusing on safety, and the requirement for HLA-A2+ status may have influenced outcome results in unknown ways, since outcome as a function of major histocompatibility complex phenotype has not been previously examined in pediatric LGGs. However, given the fact that all patients had tumors that had grown despite 2 or more prior therapies and had disease progression at study entry, the observation of 5 objective responses in this cohort, 4 of which were sustained, is of significant interest. The fact that 3 responding patients remain progression free even after completion of the vaccine regimen suggests that this approach may have long-term effects on the tumor–host interaction.
These results are nominally comparable or superior to those from several other recent trials for children who have progressed after prior “front-line” chemotherapy regimens, such as in the Children's Oncology Group (COG) study of temozolomide7 and the Pediatric Brain Tumor Consortium (PBTC) 018 study of lenalidomide,9 each of which generated sufficient interest that they formed a basis for subsequent cooperative group trials for these tumors—ACNS0223 and ACNS1022, respectively. In the COG temozolomide study, 1 of 21 (4.8%) evaluable children with low-grade astrocytomas had a PR.7 Nine children (41%) were progression free for at least one year. In PBTC 018, two of 26 (7.7%) low-grade astrocytoma patients demonstrated PRs, both of whom discontinued therapy because of drug-induced myelosuppression.9 The 12-month PFS rate was 67% ± 13%. In the more recent PBTC 022 study of bevacizumab and irinotecan, sustained PRs were seen in 2 of 35 patients (5.7%),49 and in the PBTC 029 study of the MAPK pathway inhibitor selumetinib, responses were reported in 8 of 38 patients (21.1%),14 which led to a subsequent Phase II trial (PBTC 029B). Combining immunotherapy with an agent, such as bevacizumab or selumetinib, which has limited immunosuppression might be a promising strategy for exploiting the potential benefits of each modality.
The frequency of immunological pseudoprogression in this cohort (2 of 14 patients) is lower than the rate previously observed in our cohort of high-grade gliomas and diffuse pontine gliomas,27 which may reflect key differences in the biology of these tumors in that the lower cellular density of LGGs may allow an immune response to occur without an accompanying transient increase in tumor size. Patient 4 with pseudoprogression had a particularly striking long-term response to the vaccine, which parallels our observations in malignant glioma cohorts and suggests that this phenomenon, when present, may predict a clinically relevant response. Since the pseudoprogression in this case was asymptomatic, the child was able to continue on vaccination and in the ensuing months had dramatic resolution of metastatic disease as well as shrinkage of the primary tumor, which has persisted for more than 57 months. Patient 14 with pseudoprogression had an extensive spinal cord tumor and did develop symptoms but, following a course of anti-inflammatory therapy, was able to resume vaccination and continue on the treatment protocol.
In summary, this trial demonstrated promising immunoreactivity and clinical responses to peptide vaccination in children with LGGs. These data support larger Phase II studies of GAA peptide-based vaccination in children with these tumors, in which feasibility will be assessed in the multi-institutional setting and efficacy will be evaluated in comparison with existing therapeutic approaches.
Funding
This study was supported by National Institutes of Health (NIH) grants R21CA149872 and P01NS40923; the University of Pittsburgh Cancer Institute (UPCI) Immunologic Monitoring and Cellular Products Laboratory, supported in part by NIH award P30CA47904; and the Pediatric Clinical and Translational Research Center, supported by the NIH through grants UL1 RR024153 and UL1TR000005. Support was also provided by grants from the Pediatric Low-Grade Glioma Initiative via the National Brain Tumor Society, and the Ellie Kavalieros Fund, the Connor′s Cure Fund, the Ian Yagoda′s Friends Foundation, and the Translational Brain Tumor Fund of the Children′s Hospital of Pittsburgh Foundation.
Acknowledgments
We gratefully acknowledge UPCI Clinical Research Services for regulatory management, Andres Salazar, Oncovir, Inc, for provision of poly-ICLC, physicians who referred their patients, and the patients and families who participated in this trial.
Conflict of interest statement. Hideho Okada is an inventor in the US Patent Application No. 60,611,797 (Utility Patent Application), “Identification of an IL-13 Receptor Alpha2 Peptide Analogue Capable of Enhancing Stimulation of Glioma-Specific CTL Response.” An exclusive licensing agreement has been completed on this application between the University of Pittsburgh and Stemline, Inc. Due to the potential conflicts of interest, Hideho Okada did not solely interpret any data in the current study. Dr Regina I. Jakacki is currently employed by AstraZeneca.
References
Author notes
The study has been presented in part at the following: Society for Neuro-Oncology, Washington, DC, November 15–18, 2012; Academy of Neurological Surgeons, Newport, CA, September 25–28, 2013; American Association of Neurological Surgeons, San Francisco, CA, April 9, 2014; American Society for Clinical Oncology Meeting, Chicago, IL, May 30, 2015; American Association of Neurological Surgeons Meeting, Chicago, IL, May 4, 2016.
- magnetic resonance imaging
- immune response
- brain tumors
- human leukocyte antigens
- antigens
- carboxymethylcellulose
- child
- epitopes
- glioma
- intramuscular injections
- lysine
- neoplasm metastasis
- pediatrics
- peptides
- poly i-c
- safety
- t-lymphocytes
- urticaria
- vaccination
- vaccines
- neoplasms
- survivin
- toxic effect
- enzyme linked immunospot assay
- immunization, childhood
- excision
- low grade glioma
- partial response