Effects of Filter-Membrane Materials on Concentrations of Trace Elements in Acidic Solutions
Abstract
:1. Introduction
2. Methodology
2.1. Reagents
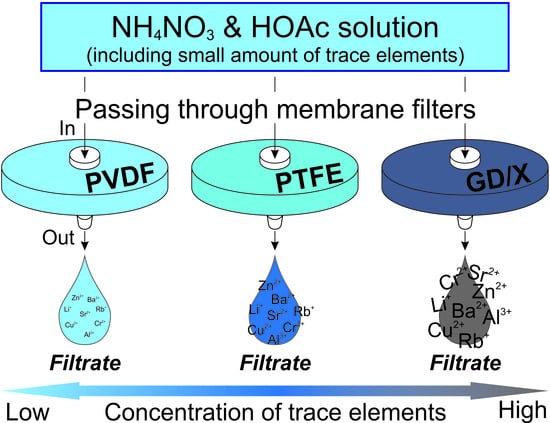
2.2. Syringe Filters
2.3. Preparation of Acid-Cleaned Filters
2.4. Sampling and Analysis
2.5. Changes in Filtrate Trace Elemental Concentrations
3. Results and Discussion
3.1. Trace Elements in NH4NO3 Filtrates
3.1.1. Filtrates from Uncleaned Filters
3.1.2. Filtrates from Acid-Cleaned Filters
3.2. Trace Elements in HOAc Filtrates
3.2.1. Filtrates from PVDF and PTFE Filters
3.2.2. Filtrates from GMF
3.3. Variation in Elemental Concentrations during Sequential Extraction
3.4. Filter Selection
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berg, T.; Røyset, O.; Steinnes, E. Blank values of trace elements in aerosol filters determined by ICP-MS. Atmos. Environ. Part A 1993, 27, 2435–2439. [Google Scholar] [CrossRef]
- Whatman Syringe Filter Collection: Superior Performance and Choice. Available online: https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/scientific/brochures-and-catalogs/brochures/ge-healthcare-whatman-syringe-filter-collection-brochure.pdf (accessed on 7 May 2020).
- Beck, A.J.; Cochran, J.K.; Sañudo-Wilhelmy, S.A. The distribution and speciation of dissolved trace metals in a shallow subterranean estuary. Mar. Chem. 2010, 121, 145–156. [Google Scholar] [CrossRef]
- Szymczycha, B.; Miotk, M.; Pempkowiak, J. Submarine groundwater discharge as a source mercury in the Bay of Puck, the southern Baltic Sea. Water Air Soil Pollut. 2013, 224, 1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golding, L.A.; McKnight, K.; Binet, M.; Adams, M.; Apte, S.C. Toxicity of dissolved and precipitated forms of barium to a frershwater alga (Chlorella sp. 12) and water flea (Ceriodaphnia dubia). Environ. Toxicol. Chem. 2018, 37, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Kurisu, M.; Tanimoto, H.; Sakaguchi, A.; Uematsu, M.; Miyamoto, C.; Takahashi, Y. Custom-made PTFE filters for ultra-clean size-fractionated aerosol sampling for trace metals. Mar. Chem. 2018, 206, 100–108. [Google Scholar] [CrossRef]
- Gäbler, H.-E.; Bahr, A.; Mieke, B. Determination of the interchangeable heavy-metal fraction in soils by isotope dilution mass spectrometry. Freseninu. J. Anal. Chem. 1999, 365, 409–414. [Google Scholar] [CrossRef]
- Pueyo, M.; López-Sánchez, J.F.; Rauret, G. Assessment of CaCl2, NaNO3 and NH4NO3 extraction procedures for the study of Cd, Cu, Pb and Zn extractability in contaminated soils. Anal. Chim. Acta 2004, 504, 217–226. [Google Scholar] [CrossRef]
- Gryschko, R.; Kuhnle, R.; Terytze, K.; Breuer, J.; Stahr, K. Soil extraction of readily soluble heavy metals and As with 1 M NH4NO3-solution evaluation of DIN 19730. J. Soils Sediments 2005, 5, 101–106. [Google Scholar] [CrossRef]
- Smoleń, S.; Sady, W.; Ledwożyw-Smoleń, I. Quantitative relations between the content of selected trace elements in soil extracted with 0.03 M CH3COOH or 1 M HCl and its total concentration in carrot storage roots. Acta Sci. Pol. Hortorum Cultus. 2010, 9, 3–12. [Google Scholar]
- Willmes, M.; McMorrow, L.; Kinsley, L.; Armstrong, R.; Aubert, M.; Eggins, S.; Falguères, C.; Maureille, B.; Moffat, I.; Grün, R. The IRHUM (Isotopic Reconstruction of Human Migration) database – bioavailable strontium isotope ratios for geochemical fingerprinting in France. Earth Syst. Sci. Data 2014, 6, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Adams, S.; Grün, R.; McGahan, D.; Zhao, J.-X.; Feng, Y.; Nguyen, A.; Willmes, M.; Quaresimin, M.; Lobsey, B.; Collard, M.; et al. A strontium isoscape of north-east Australia for human provenance and repatriation. Geoarchaeology 2019, 34, 231–251. [Google Scholar] [CrossRef]
- WhatmanTM Syringe Filter and Filter Vial Collection: Outstanding Performance and Choice. Available online: https://beta-static.fishersci.com/content/dam/fishersci/en_EU/promotions/GEHealthcareLifeSciences/Whatman_Syringe_Filter_and_Filter_Vial_Brochure.pdf (accessed on 30 June 2020).
- Hedberg, Y.; Herting, G.; Wallinder, I.O. Risks of using membrane filtration for trace metal analysis and assessing the dissolved metal fraction of aqueous media – A study on zinc, copper and nickel. Environ. Pollut. 2011, 159, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Raush, N.; Ukonmaanaho, L.; Nieminen, T.M.; Krachler, M.; Le Roux, G.; Shotyk, W. Evaluation of samplers and filter materials for the establishment of trace metal concentration profiles in peat bog porewaters using inductively coupled plasma-mass spectrometry. Anal. Chim. Acta 2006, 558, 201–210. [Google Scholar] [CrossRef]
- Song, B.-Y.; Ryu, J.-S.; Shin, H.S.; Lee, K.-S. Determination of the source of bioavailable Sr using 87Sr/86Sr tracers: A case study of hot pepper and rice. J. Agric. Food Chem. 2014, 62, 9232–9238. [Google Scholar] [CrossRef] [PubMed]
- Hoogewerff, J.A.; Reimann, C.; Ueckermann, H.; Frei, R.; Frei, K.M.; van Aswegen, T.; Stirling, C.; Reid, M.; Clayton, A.; Ladenberger, A.; et al. Bioavailable 87Sr/86Sr in European soils: A baseline for provenancing studies. Sci. Total Environ. 2019, 672, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Elements | Primary Solution | Uncleaned Filter | Acid-Cleaned Filter | ||||
|---|---|---|---|---|---|---|---|
| 1 PVDF (n = 30) | 2 PTFE (n = 30) | 3 GD/X (n = 30) | PVDF (n = 30) | PTFE (n = 30) | GD/X (n = 30) | ||
| Avg. ± SD | Avg. ± SD | Avg. ± SD | Avg. ± SD | Avg. ± SD | Avg. ± SD | Avg. ± SD | |
| Ammonium nitrate (NH4NO3) | |||||||
| Al | 4.01 ± 1.67 | BDL | 0.27 ± 1.01 | BDL | 1.62 ± 4.72 | 1.57 ± 2.65 | 3.00 ± 8.37 |
| Cr | 0.50 ± 0.50 | 0.50 ± 0.13 | 0.67 ± 0.20 | 0.92 ± 0.80 | 0.80 ± 0.35 | 0.84 ± 0.24 | 1.17 ± 0.72 |
| Mn | 1.09 ± 0.06 | 0.22 ± 0.06 | 0.46 ± 0.21 | 0.36 ± 0.14 | 0.62 ± 0.07 | 0.93 ± 0.29 | 1.34 ± 0.93 |
| Zn | 8.74 ± 0.63 | 231 ± 46.0 | 175 ± 76.1 | 686 ± 537 | 10.1 ± 10.2 | 5.21 ± 2.93 | 3071 ± 3832 |
| Li | 18.4 ± 0.25 | 12.9 ± 0.31 | 17.4 ± 3.69 | 13.5 ± 0.54 | 19.0 ± 0.57 | 19.4 ± 0.85 | 18.3 ± 2.59 |
| Cu | 7.16 ± 0.57 | 5.31 ± 0.41 | 6.74 ± 1.16 | 5.88 ± 0.78 | 6.76 ± 0.45 | 8.37 ± 1.26 | 9.88 ± 3.01 |
| Mo | 56.1 ± 1.52 | 17.7 ± 0.45 | 25.7 ± 7.21 | 19.1 ± 1.05 | 28.4 ± 0.88 | 30.0 ± 1.41 | 27.6 ± 5.98 |
| Ba | 0.59 ± 0.09 | 32.3 ± 1.04 | 48.2 ± 13.1 | 1492 ± 1633 | 66.1 ± 1.34 | 93.4 ± 18.8 | 6564 ± 9687 |
| Co | 4 BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Ni | BDL | 0.75 ± 0.27 | 0.96 ± 0.37 | 0.86 ± 0.45 | 1.90 ± 0.21 | 2.62 ± 0.67 | 1.57 ± 0.41 |
| Rb | BDL | BDL | 0.11 ± 0.10 | 1.45 ± 1.97 | BDL | 0.07 ± 0.37 | 1.59 ± 2.16 |
| Sr | BDL | 0.24 ± 0.09 | 0.32 ± 0.11 | 11.0 ± 8.96 | BDL | 0.11 ± 0.50 | 27.9 ± 36.3 |
| Cd | BDL | 0.25 ± 0.03 | 0.11 ± 0.13 | 0.26 ± 0.04 | BDL | 0.01 ± 0.04 | BDL |
| Cs | BDL | BDL | BDL | BDL | BDL | BDL | 0.29 ± 0.46 |
| Pb | BDL | BDL | BDL | 0.25 ± 0.38 | BDL | 0.02 ± 0.10 | BDL |
| U | BDL | BDL | BDL | BDL | BDL | 0.01 ± 0.06 | BDL |
| Acetic acid (HOAc) | |||||||
| Al | 1.15 ± 0.17 | 0.95 ± 0.66 | 0.12 ± 0.51 | 306 ± 236 | 9.88 ± 3.40 | 6.92 ± 1.95 | 311 ± 228 |
| Cr | 3.32 ± 0.37 | 4.44 ± 0.34 | 4.54 ± 0.46 | 5.54 ± 1.19 | 5.86 ± 0.70 | 5.38 ± 0.64 | 8.47 ± 3.08 |
| Mn | 0.07 ± 0.12 | BDL | BDL | 0.62 ± 0.60 | 0.53 ± 0.15 | 0.41 ± 0.18 | 1.56 ± 2.11 |
| Zn | 4.74 ± 0.96 | 3.83 ± 2.80 | 7.56 ± 18.1 | 1675 ± 2044 | 68.2 ± 33.4 | 84.4 ± 6.11 | 6450 ± 12,129 |
| Li | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Cu | BDL | BDL | BDL | 0.48 ± 1.04 | 0.70 ± 0.43 | 1.04 ± 0.21 | 2.21 ± 3.79 |
| Mo | BDL | BDL | BDL | 0.05 ± 0.16 | BDL | 0.24 ± 0.47 | BDL |
| Ba | BDL | BDL | BDL | 3188 ± 4365 | 0.51 ± 0.35 | 0.64 ± 0.34 | 7353 ± 14,568 |
| Co | BDL | BDL | BDL | BDL | BDL | BDL | BDL |
| Ni | BDL | BDL | BDL | 0.21 ± 0.58 | BDL | BDL | BDL |
| Rb | BDL | BDL | BDL | 0.56 ± 1.38 | BDL | BDL | 1.08 ± 1.96 |
| Sr | BDL | BDL | BDL | 24.2 ± 31.7 | BDL | 0.11 ± 0.49 | 42.0 ± 92.8 |
| Cd | BDL | BDL | BDL | BDL | BDL | 0.02 ± 0.13 | BDL |
| Cs | BDL | BDL | BDL | 0.08 ± 0.21 | BDL | 0.01 ± 0.07 | BDL |
| Pb | BDL | BDL | BDL | 0.32 ± 0.64 | BDL | BDL | BDL |
| U | BDL | BDL | BDL | 0.02 ± 0.02 | BDL | BDL | BDL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, W.-J.; Shin, H.-S.; Hwang, J.-H.; Lee, K.-S. Effects of Filter-Membrane Materials on Concentrations of Trace Elements in Acidic Solutions. Water 2020, 12, 3497. https://doi.org/10.3390/w12123497
Shin W-J, Shin H-S, Hwang J-H, Lee K-S. Effects of Filter-Membrane Materials on Concentrations of Trace Elements in Acidic Solutions. Water. 2020; 12(12):3497. https://doi.org/10.3390/w12123497
Chicago/Turabian StyleShin, Woo-Jin, Hyung-Seon Shin, Ji-Hun Hwang, and Kwang-Sik Lee. 2020. "Effects of Filter-Membrane Materials on Concentrations of Trace Elements in Acidic Solutions" Water 12, no. 12: 3497. https://doi.org/10.3390/w12123497