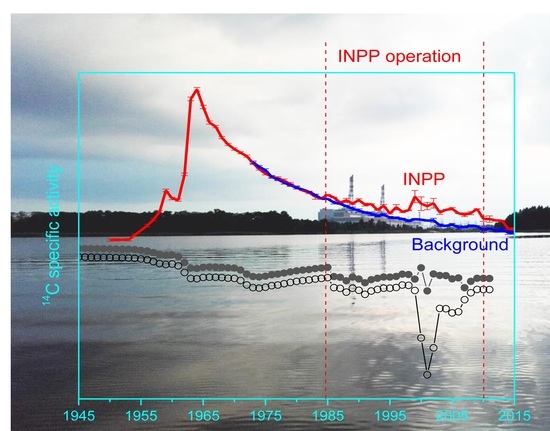
Modeling of 14C Vertical Distribution in Bottom Sediments of the Ignalina Nuclear Power Plant Cooling Reservoir
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site Description
2.2. Model Description
3. Results and Discussion
3.1. Model Assumptions
3.2. Evaluation of 14C Basic Trend in Bottom Sediments
3.3. Change in Assimilation Rates λ and DIC Discharges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- IAEA. Management of waste containing tritium and Carbon-14. In International Atomic Energy Agency Technical Report 421; IAEA: Vienna, Austria, 2004; ISBN 92–0–114303–6. [Google Scholar]
- NCRP. Carbon-14 in the environment. In NCRP Report No. 81; NCRP: Bethesda, MD, USA, 1984. [Google Scholar]
- Kunz, C. Carbon-14 discharge at three light-water reactors. Health Phys. 1985, 49, 25–35. [Google Scholar] [CrossRef]
- Molnár, M.; Bujtás, T.; Svingor, É.; Futó, I.; Světlík, I. Monitoring of atmospheric excess 14C around Paks nuclear power plant, Hungary. Radiocarbon 2007, 49, 1031–1043. [Google Scholar] [CrossRef] [Green Version]
- Sohn, W.; Kang, D.W.; Kim, W.S. An estimate of carbon-14 inventory at Wolsong Nuclear Power Plant in the Republic of Korea. J. Nucl. Sci. Technol. 2003, 40, 604–613. [Google Scholar] [CrossRef]
- Stenström, K.; Erlandsson, B.; Mattsson, S.; Thornberg, C.; Hellborg, R.; Kiisk, M. 14C emission from Swedish nuclear power plants and its effect on the 14C levels in the environment. In Internal Report LUNDFD6/(NFFR-3079); Lund University: Lund, Sweden, 2000; pp. 1–44. [Google Scholar]
- Uchrin, G.; Hertelendi, E.; Volent, G.; Slavik, O.; Morávek, J.; Kobal, I.; Vokal, B. 14C Measurements at PWR-Type Nuclear Power Plants in Three Middle European Countries. Radiocarbon 1997, 40, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Veres, M.; Hertelendi, E.; Uchrin, G.; Csaba, E.; Barnabás, I.; Ormai, P.; Volent, G.; Futó, I. Concentration of radiocarbon and its chemical forms in gaseous effluents, environmental air, nuclear waste and primary water of a pressurized water reactor power plant in Hungary. Radiocarbon 1995, 37, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Cook, G.T.; Cresswell, A.J.; Dunbar, E.; Freeman, S.P.H.T.; Hastie, H.; Hou, X.; Jacobsson, P.; Naysmith, P.; Sanderson, D.C.W.; et al. 14C levels in the vicinity of the Fukushima Dai-ichi Nuclear Power Plant prior to the 2011 accident. J. Environ. Radioact. 2016, 157, 90–96. [Google Scholar] [CrossRef]
- Yim, M.S.; Caron, F. Life cycle and management of carbon-14 from nuclear power generation. Prog. Nucl. Energy 2006, 48, 2–36. [Google Scholar] [CrossRef]
- Gaiko, V.B.; Korablev, N.A.; Solov’ev, E.N.; Trosheva, T.I.; Shamov, V.P.; Umanets, M.P.; Shcherbina, V.G. Discharge of 14C by nuclear power stations with RBMK-1000 reactors. Sov. At. Energy 1986, 59, 703–705. [Google Scholar] [CrossRef]
- Konstantinov, E.A.; Korablev, N.A.; Solov’ev, E.N.; Shamov, V.P.; Fedorov, V.L.; Litvinov, A.M. 14C emission from RBMK-1500 reactors and features determining it. Sov. At. Energy 1989, 66, 77–79. [Google Scholar] [CrossRef]
- Barisevičiūtė, R.; Maceika, E.; Ežerinskis, Ž.; Šapolaitė, J.; Butkus, L.; Mažeika, J.; Rakauskas, V.; Juodis, L.; Steponėnas, A.; Druteikienė, R.; et al. Distribution of radiocarbon in sediments of the cooling pond of RBMK type Ignalina Nuclear Power Plant in Lithuania. PLoS ONE 2020, 15, e0237605. [Google Scholar] [CrossRef]
- Mažeika, J. Carbon-14 in terrestrial and aquatic environment of Ignalina nuclear power plant: Sources of production, releases and dose estimates. In Nuclear Power; Tsvetjov, P.V., Ed.; InTech: Rijeka, Croatia, 2010; pp. 293–310. [Google Scholar]
- Ežerinskis, Ž.; Šapolaite, J.; Pabedinskas, A.; Juodis, L.; Garbaras, A.; Maceika, E.; Druteikiene, R.; Lukauskas, D.; Remeikis, V. Annual variations of 14C concentration in the tree rings in the vicinity of Ignalina Nuclear Power Plant. Radiocarbon 2018, 60, 1227–1236. [Google Scholar] [CrossRef]
- Pabedinskas, A.; Maceika, E.; Šapolaitė, J.; Ežerinskis, Ž.; Juodis, L.; Butkus, L.; Bučinskas, L.; Remeikis, V. Assessment of the Contamination by 14C Airborne Releases in the Vicinity of the Ignalina Nuclear Power Plant. Radiocarbon 2019, 61, 1185–1197. [Google Scholar] [CrossRef]
- Mažeika, J.; Taminskas, J. Evaluation of recent sedimentation rates in the lakes of East Lithuania based on radioisotope dating. Geogr. Metraštis 2005, 38, 167–173. [Google Scholar]
- Marčiulioniene, D.; Montvydiene, D.; Kazlauskiene, N.; Kesminas, V. Changes in macrophytes and fish communities in the cooler of Ignalina NuclearPower Plant (1988–2008). J. Environ. Eng. Landsc. Manag. 2011, 19, 21–33. [Google Scholar] [CrossRef]
- Kleber, M.; Lehmann, J. Humic substances extracted by alkali are invalid proxies for the dynamics and functions of organic matter in terrestrial and aquatic ecosystems. J. Environ. Qual. 2019, 48, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Donahue, D.J.; Linick, T.W.; Jull, A.J.T. Isotope-ratio and background corrections for accelerator mass spectrometry radiocarbon measurements. Radiocarbon 1990, 32, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Stuiver, M.; Polach, H.A. Reporting of 14C data. Radiocarbon 1977, 19, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Nedveckaite, T.; Marciulioniene, D.; Mazeika, J.; Paskauskas, R. Radiological and environmental effects in Ignalina nuclear power plant cooling pond—Lake Druksiai: From plant put in operation to shut down period of time. In Nuclear Power—Operation, Safety and Environment; Tsvetkov, P., Ed.; IntechOpen: Rijeka, Croatia, 2011; ISBN 978-953-307-507-5. [Google Scholar]
- Lamb, A.L.; Leng, M.J.; Mohammed, M.U.; Lamb, H.F. Holocene climate and vegetation change in the Main Ethiopian Rift Valley, inferred from the composition (C/N and δ13C) of lacustrine organic matter. Quat. Sci. Rev. 2004, 23, 881–891. [Google Scholar] [CrossRef]
- Leng, M.J.; Marshall, J.D. Palaeoclimate interpretation of stable isotope data from lake sediment archives. Quat. Sci. Rev. 2004, 23, 811–831. [Google Scholar] [CrossRef] [Green Version]
- Cloern, J.E.; Canuel, E.A.; Harris, D. Stable carbon and nitrogen isotope composition of aquatic and terrestrial plants of the San Francisco Bay estuarine system. Limnol. Oceanogr. 2002, 47, 713–729. [Google Scholar] [CrossRef]
- Contreras, S.; Werne, J.P.; Araneda, A.; Urrutia, R.; Conejero, C.A. Organic matter geochemical signatures (TOC, TN, C/N ratio, δ13C and δ15N) of surface sediment from lakes distributed along a climatological gradient on the western side of the southern Andes. Sci. Total Environ. 2018, 630, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.R.; Kragh, T.; Martinsen, K.T.; Kristensen, E.; Sand-Jensen, K. The carbon pump supports high primary production in a shallow lake. Aquat. Sci. 2019, 81, 1–11. [Google Scholar] [CrossRef]
- Orion-Jedrysek, M.; Kurasiewicz, M.; Trojanowska, A.; Lewicka, D.; Omilanowska, A.; Kalżuny, A.; Izydorczyk, K.; Drzewicki, W.; Zalewski, M. Diurnal variations in carbon isotope composition of dissolved inorganic carbon (DIC) in a freshwater dam reservoir. Ecohydrol. Hydrobiol. 2006, 6, 53–59. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Shirokova, L.S. Diurnal variations of dissolved and colloidal organic carbon and trace metals in a boreal lake during summer bloom. Water Res. 2013, 47, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Schindler, D.W.; Fee, E.J. Diurnal variation of dissolved inorganic carbon and its use in estimating primary production and CO2 invasion in Lake 227. J. Fish. Res. Board Canada 1973, 30, 1501–1510. [Google Scholar] [CrossRef]
- Waldron, S.; Scott, E.M.; Soulsby, C. Stable isotope analysis reveals lower-order river dissolved inorganic carbon pools are highly dynamic. Environ. Sci. Technol. 2007, 41, 6156–6162. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiang, M.; Jin, Y.; Liu, L.; Zhou, A.; Zhang, J. Influence of aquatic plant photosynthesis on the reservoir effect of genggahai lake, northeastern qinghai-tibetan plateau. Radiocarbon 2018, 60, 561–569. [Google Scholar] [CrossRef]
- Šarauskienė, D. Thermal regime database of Ignalina nuclear power plant cooler-Lake Drukšiai. Environ. Monit. Assess. 2002, 79, 1–12. [Google Scholar] [CrossRef]
- Steinberg, C.E.W.; Paul, A.; Pflugmacher, S.; Meinelt, T.; Klöcking, R.; Wiegand, C. Pure humic substances have the potential to act as xenobiotic chemicals—A review. Fresenius Environ. Bull. 2003, 12, 391–401. [Google Scholar]
- Wang, F.; Yi, X.; Qu, H.; Chen, L.; Liu, D.; Wang, P.; Zhou, Z. Enantioselective accumulation, metabolism and phytoremediation of lactofen by aquatic macrophyte Lemna minor. Ecotoxicol. Environ. Saf. 2017, 143, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, J.; Antoš, V.; Micalizzi, G.; Dresler, S.; Hrabák, P.; Mondello, L. Accumulation and toxicity of organochlorines in green microalgae. J. Hazard. Mater. 2018, 347, 168–175. [Google Scholar] [CrossRef]
- Dhir, B. Chapter 21-Green technologies for the removal of agrochemicals by aquatic plants. In Treatment and Remediation; Prasad, M.N.V., Ed.; Butterworth-Heinemann Ltd: Oxford, UK, 2020; pp. 569–591. ISBN 9780081030172. [Google Scholar]
- Bhatia, M.; Goyal, D. Analyzing remediation potential of wastewater through wetland plants: A review. Environ. Prog. Sustain. Energy 2014, 33, 9–27. [Google Scholar] [CrossRef]
- Hou, J.; D’Andrea, W.J.; Liu, Z. The influence of 14C reservoir age on interpretation of paleolimnological records from the Tibetan Plateau. Quat. Sci. Rev. 2012, 48, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Ascough, P.L.; Cook, G.T.; Hastie, H.; Dunbar, E.; Church, M.J.; Einarsson, A.; McGovern, T.H.; Dugmore, A.J. An Icelandic freshwater radiocarbon reservoir effect: Implications for lacustrine 14C chronologies. Holocene 2011, 21, 1073–1080. [Google Scholar] [CrossRef] [Green Version]
- Mažeika, J.; Taminskas, J.; Paškauskas, R.; Bodoyan, A.; Baghdassaryan, H.; Tozalakyan, P.; Davtyan, V.; Grillot, J.-C.; Travi, Y. Ecohydrological evolution in the catchment of Lake Drūkšiai, Lithuania, under anthropogenic pressure. Ekologija 2006, 4, 40–50. [Google Scholar]





| Symbol | Value | Units | Name |
|---|---|---|---|
| 3.31 × 10−3 | year−1 | Decomposition/dissolution constant of alkali-soluble fraction | |
| 2.58 × 10−3 | year−1 | Decomposition/dissolution constant of alkali-insoluble fraction | |
| 1.00 | year−1 | Assimilation rate of alkali-insoluble fraction to alkali-soluble | |
| 0.35 | year−1 | Assimilation rate of alkali- soluble fraction to alkali-insoluble | |
| Pollution event 2000–2009 (A) | |||
| 1.00 | year−1 | Assimilation rate of alkali-insoluble fraction to alkali-soluble | |
| 0.35; with exception of 0.29 during the period of 2000–2006 | year−1 | Assimilation rate of alkali- soluble fraction to alkali-insoluble | |
| Pollution event 2000–2009 (B) | |||
| 1.00; with exception of 1.27 during the period 2000–2002 then 14C pollution in DIC form was added | year−1 | Assimilation rate of alkali-insoluble fraction to alkali-soluble | |
| 0.35; with exception of 0.29 during the period of 2000–2006 | year−1 | Assimilation rate of alkali-soluble fraction to alkali-insoluble | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barisevičiūtė, R.; Maceika, E.; Juodis, L.; Pabedinskas, A.; Šapolaitė, J.; Ežerinskis, Ž.; Butkus, L.; Remeikis, V. Modeling of 14C Vertical Distribution in Bottom Sediments of the Ignalina Nuclear Power Plant Cooling Reservoir. Water 2021, 13, 1397. https://doi.org/10.3390/w13101397
Barisevičiūtė R, Maceika E, Juodis L, Pabedinskas A, Šapolaitė J, Ežerinskis Ž, Butkus L, Remeikis V. Modeling of 14C Vertical Distribution in Bottom Sediments of the Ignalina Nuclear Power Plant Cooling Reservoir. Water. 2021; 13(10):1397. https://doi.org/10.3390/w13101397
Chicago/Turabian StyleBarisevičiūtė, Rūta, Evaldas Maceika, Laurynas Juodis, Algirdas Pabedinskas, Justina Šapolaitė, Žilvinas Ežerinskis, Laurynas Butkus, and Vidmantas Remeikis. 2021. "Modeling of 14C Vertical Distribution in Bottom Sediments of the Ignalina Nuclear Power Plant Cooling Reservoir" Water 13, no. 10: 1397. https://doi.org/10.3390/w13101397