Evaluation of Element Mobility in River Sediment Using Different Single Extraction Procedures and Assessment of Probabilistic Ecological Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Sample Preparation
2.3. Sample Extractions and Measurement Using ICP-OES
2.4. Measurement Using ICP-OES
2.5. Pollution Risk Assessment and Monte Carlo Simulation
2.6. Determination of Magnetic Susceptibility
3. Results and Discussion
3.1. Quality Control and Assurance
3.2. Discussion about Extracted Elements Contents by Different Extraction Agents
3.3. Discussion of Concentration Ratios of Studied Elements Using Different Extraction Agents with Results of Amounts of Elements Extracted by the BCR Sequential Extraction Procedure (Total Element Content)
3.4. Correlation Analysis
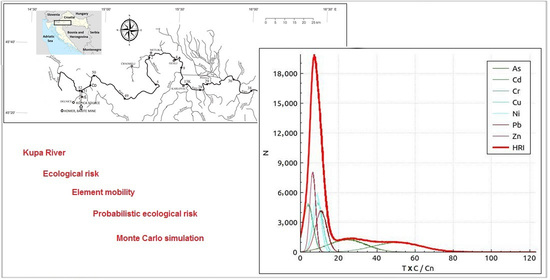
3.5. Ecological Risk Assessment of Potentially Toxic Elements Using Monte Carlo Simulation
3.6. Magnetic Susceptibility (MS) Measurements vs. Element Concentrations in Different Dissolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davutluoglu, O.I.; Seckin, G.; Ersu, C.B.; Yilmaz, T.; Sari, B. Heavy metal content and distribution in surface sediments of the Seyhan River, Turkey. J. Environ. Manag. 2011, 92, 2250–2259. [Google Scholar] [CrossRef]
- Zahra, A.; Hashmi, M.Z.; Malik, R.N.; Ahmed, Z. Enrichment and geo-accumulation of heavy metals and risk assessment of sediments of the Kurang Nallah—Feeding tributary of the Rawal Lake Reservoir, Pakistan. Sci. Total Environ. 2014, 470–471, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Ottesen, R.T.; Bogen, J.; Bølviken, B.; Volden, T. Overbank sediment: A representative sampling medium for regional geochemical mapping. J. Geochem. Explor. 1989, 32, 257–277. [Google Scholar] [CrossRef]
- Cappuyns, V. A critical evaluation of single extractions from the SMT program to determine trace element mobility in sediments. Appl. Environ. Soil Sci. 2012, 672914. [Google Scholar] [CrossRef] [Green Version]
- Groenenberg, J.E.; Römkens, P.F.A.M.; Zomeren, A.V.; Rodrigues, S.M.; Comans, R.N.J. Evaluation of the single dilute (0.43 M) nitric acid extraction to determine geochemically reactive elements in Soil. Environ. Sci. Technol. 2017, 51, 2246–2253. [Google Scholar] [CrossRef] [Green Version]
- Antoniadis, V.; Shaheen, S.M.; Levizou, E.; Shahid, M.; Niazi, N.K.; Vithanage, M.; Ok, Y.S.; Bolan, N.; Rinklebe, J. A critical prospective analysis of the potential toxicity of trace element regulation limits in soils worldwide: Are they protective concerning health risk assessment?-A review. Environ. Internat. 2019, 127, 819–847. [Google Scholar] [CrossRef]
- Kastori, R.R.; Petrović, M.; Petrović, N.M.; Štrbac, D. Effect of heavy metals on water relations in plants. Zb. Matice Srp. Prir. Nauke 1995, 88, 5–17. [Google Scholar]
- Abollino, O.; Malandrino, M.; Giacomino, A.; Mentasti, E. The role of chemometrics in single and sequential extraction assays: A review Part I. Extraction procedures, uni- and bivariate techniques and multivariate variable reduction techniques for pattern recognition. Anal. Chim. Acta. 2011, 688, 104–121. [Google Scholar] [CrossRef]
- Cuvier, A.; Leleyter, L.; Probst, A.; Probst, J.-L.; Prunier, J.; Poucelot, L.; Le Roux, G.; Lemoine, M.; Reinert, M.; Baraud, F. Why comparison between different chemical extraction procedures is necessary to better assess the metals availability in sediments? J. Geochem. Explor. 2021, 225, 106762. [Google Scholar] [CrossRef]
- Pantović Spajić, K.; Sakan, S.; Đorđević, D.; Šoštarić, T.; Lopičić, Z.; Janićijević, A.; Stojanović, K. Comparison of extraction agents for metal determination in sediments from artificial lakes and rivers in Serbia. Acta Period. Technol. 2019, 50, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Milićević, T.; Relić, D.; Škrivanj, S.; Tešić, Ž.; Popović, A. Assessment of major and trace element bioavailability in vineyard soil applying different single extraction procedures and pseudo-total digestion. Chemosphere 2017, 171, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Pueyo, M.; López-Sánchez, J.F.; Rauret, G. Assessment of CaCl2, NaNO3 and NH4NO3 extraction procedures for the study of Cd, Cu, Pb and Zn extractability in contaminated soils. Anal. Chim. Acta 2004, 504, 217–226. [Google Scholar] [CrossRef]
- Zhao, L.; Mi, D.; Wang, L.; Sun, Y. Ecological risk assessment and sources of heavy metals in sediment from Daling River basin. Environ. Sci. Pollut. Res. 2015, 22, 5975–5984. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Song, S.; An, S.; Liu, E. Ecological risk assessment of potentially toxic elements (PTEs) in the soil-plant system after reclamation of dredged sediment. Environ. Sci. Poll. Res. 2018, 25, 29181–29191. [Google Scholar] [CrossRef] [PubMed]
- Poulter, S.R. Monte Carlo Simulation in Environmental Risk Assessment—Science, Policy and Legal Issues. RISK 1998, 9, 7. [Google Scholar]
- Frančišković-Bilinski, S. Barium anomaly in Kupa River drainage basin. J. Geochem. Explor. 2006, 88, 106–109. [Google Scholar] [CrossRef]
- Frančišković-Bilinski, S.; Bilinski, H.; Grbac, R.; Žunić, J.; Nečemer, M.; Hanžel, D. Multidisciplinary work on barium contamination of the karstic upper Kupa River drainage basin (Croatia and Slovenia); calling for watershed management. Environ. Geochem. Health 2007, 29, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Frančišković-Bilinski, S.; Bhattacharya, A.K.; Bilinski, H.; Bhattacharya, B.D.; Mitra, A.; Sarkar, S.K. Fluvial geomorphology of the Kupa River drainage basin, Croatia: A perspective of its application in river management and pollution studies. Zeitschrift für Geomorphologie 2012, 56, 93–119. [Google Scholar] [CrossRef]
- Sakan, S.; Frančišković-Bilinski, S.; Đorđević, D.; Popović, A.; Škrivanj, S.; Bilinski, H. Geochemical fractionation and risk assessment of potentially toxic elements in sediments from Kupa River, Croatia. Water 2020, 12, 2024. [Google Scholar] [CrossRef]
- Biondić, B.; Biondić, R.; Kapelj, S. Protection of the Karst aquifers in the river Kupa catchment area and sustainable development. RMZ Mater. Geoenv. 2003, 50, 33–36. [Google Scholar]
- Cappuyns, V.; Swennen, R.; Verhulst, A. Assessment of Heavy Metal Mobility in Dredged Sediments: Porewater Analysis, Single and Sequential Extractions. Soil Sedim. Cont. 2006, 15, 1–18. [Google Scholar] [CrossRef]
- Sihlahla, M.; Mouri, H.; Nomngongo, P.N. Assessment of bioavailability and mobility of major and trace elements in agricultural soils collected in Port St Johns, Eastern Cape, South Africa using single extraction procedures and pseudo-total digestion. J. Environ. Health Sci. Eng. 2020, 18, 1615–1628. [Google Scholar] [CrossRef]
- Sakan, S.; Popović, A.; Škrivanj, S.; Sakan, N.; Đorđević, D. Comparison of single extraction procedures and the application of an index for the assessment of heavy metal bioavailability in river sediments. Environ. Sci. Pollut. Res. 2016, 23, 21485–21500. [Google Scholar] [CrossRef]
- Šestinová, O.; Hančuľák, J.; Brehuv, J.; Fedorová, E. The mobility of heavy metals in sediments using the sequential extraction method. In Proceedings of the Conference Materials, 4th European Conference Bioremediation, Chania Crete, Greece, 3–6 September 2008. [Google Scholar]
- Šmejkalová, M.; Mikanová, O.; Borůvka, L. Effects of heavy metal concentrations on biological activity of soil micro-organisms. Plant Soil Environ. 2003, 49, 321–326. [Google Scholar] [CrossRef]
- Qu, C.; Li, B.; Wu, H.; Wang, S.; Li, F. Probabilistic ecological risk assessment of heavy metals in sediments from China’s major aquatic bodies. Stoch. Environ. Res. Risk Assess. 2016, 30, 271–282. [Google Scholar] [CrossRef]
- Wu, H.; Li, B.; Qu, C.; Wang, S.; Wan, W.; Zhou, J. A Method for Determining Ecological Risks of Heavy Metal Pollution in River and Lake Sediments. U.S. Patent 20160110835A1, 21 April 2016. [Google Scholar]
- Håkanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Shen, Z.; Niu, J.; Tang, Z. Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze river catchment of Wuhan, China. J. Hazard. Mater. 2009, 166, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Pavlů, L.; Drábek, O.; Borůvka, L.; Nikodem, A.; Němeček, K. Degradation of forest soils in the vicinity of an industrial zone. Soil Water Res. 2015, 10, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Carter, M.R. Soil Sampling and Methods of Analysis; Lewis Publishers: Boca Raton, FL, USA; Canadian Society of Soil Science: Pinawa, MB, Canada, 1993. [Google Scholar]
- Podlesáková, E.; Nemecek, J.; Vácha, R. Mobility and Bioavailability of Trace Elements in Soils. In Trace Elements in Soil: Bioavailability, Flux, and Transfer; Iskandar, I.K., Kirkham, M.B., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2001. [Google Scholar]
- Sabienë, N.; Brazauskienë, D.M.; Rimmer, D. Determination of heavy metal mobile forms by different extraction methods. Ekologija 2004, 1, 36–41. [Google Scholar]




| Number | Label | Fraction (μm) | Clarification of the Locality |
|---|---|---|---|
| 1 | DN-2 | <63 | Dobra River, upper course, only about 5 km downstream from the source, between the villages of Gornja and Donja Dobra |
| 2 | IŠ | <63 | the river Kupica, approximately 1.5 km upstream from its confluence with the Kupa |
| 3 | K-4 | <63 | Kupa River in Ozalj, right bank, just below the dam of the Ozalj hydroelectric power plant |
| 4 | K-2 | <63 | Kupa River in Jurovo, right bank, about 250 m upstream from the mouth of the river Lahinja |
| 5 | 38 | <63 | Kupa River in the settlement Sunčani brijeg, about 500 m downstream, left bank |
| 6 | ČD | <63 | Kupa River, right bank, the village of Čedanj, located approximately 5 km downstream from Brod na Kupi |
| 7 | 44 | <63 | Kupa River, Sisak, right bank, about 2 km before the confluence of the Odra and Kupa |
| 8 | 51 | <63 | Kupica River in Brod na Kupi, just before the confluence with the Kupa River |
| 9 | IŠ | 63–2000 | Kupica River, approximately 1.5 km upstream from its confluence with the Kupa River |
| 10 | 44 | 63–2000 | Kupa River, Sisak, Zibel beach, right bank, about 2 km before the confluence of the Odra and Kupa |
| 11 | 45 | 63–2000 | Kupa River, Letovanić, left bank |
| 12 | K-2 | 63–2000 | Kupa River in Jurovo, right bank, about 250 m upstream from Lahinja River inflow |
| 13 | 51 | 63–2000 | Kupica River in Brod na Kupi, just before the confluence with the Kupa River |
| 14 | K-5 | 63–2000 | Kupa River, Jakovci Netretićki, in the part where Kupa passes from its upper to middle course, was sampled from the right bank near the waterfall below the village |
| 15 | K-9 | 63–2000 | Kupa River, between the villages of Levkušje and Zorkovac, right bank, about 3.5 km upstream from the Dobra River inflow |
| 16 | 38 | 63–2000 | Kupa River in the settlement Sunčani breg, about 500 m downstream, left bank |
| 17 | 52 | 63–2000 | Kupa River in Brod na Kupi, right bank, sample taken about 100 m upstream from the mouth of the Kupica River |
| 18 | ČD | 63–2000 | Kupa River, right bank, the village of Čedanj, located approximately 5 km downstream from Brod na Kupi |
| 19 | 28 | 63–2000 | Kupa River, Donja Rečica, left bank, on the location right across from Brođani |
| 20 | 41 | 63–2000 | Kupa River, Petrinja, town beach, 800 m downstream from the bridge on the main road from Petrinja to Zagreb, right bank |
| 21 | 49 | 63–2000 | Kupa River, the upper course of the Kupa in Gorski Kotar, about 1 km upstream from the village of Severin na Kupi |
| 22 | 29 | 63–2000 | Kupa River, Zamršlje, about 7 km downstream from point 28 |
| 23 | 36 | 63–2000 | Kupa River, Lijevo Sredičko, left bank, lower course, 12 km upstream from Pokupsko |
| 24 | K-4 | 63–2000 | Kupa River in Ozalj, right bank, just below the dam of the Ozalj hydroelectric power plant |
| 25 | DN-2 | 63–2000 | Dobra River, upper course, only about 5 km downstream from the source, between the villages of Gornja and Donja Dobra |
| Cd | Cr | Cu | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|
| Obtained | 0.48 ± 0.03 | 0.41 ± 0.06 | 1.4 ± 0.1 | 1.6 ± 0.1 | BDL 1 | 8.1 ± 0.5 |
| Indicative | 0.45 ± 0.05 | 0.35 ± 0.09 | 1.2 ± 0.4 | 1.4 ± 0.2 | <0.06 | 8.3 ± 0.7 |
| Recovery | 106.7% | 117.1% | 116.7% | 114.3% | / | 97.6% |
| Fraction: <63 μm | Fraction: 63–2000 μm | |||||||
|---|---|---|---|---|---|---|---|---|
| [mg kg−1] | CaC 1 | AA | NA | BCR | CaC | AA | NA | BCR |
| Al | Al | |||||||
| Mean | 0.092 | 1.60 | 914 | 9836 | 0.140 | 0.80 | 255 | 3791 |
| Max | 0.144 | 6.55 | 1200 | 11,865 | 0.772 | 3.95 | 418 | 7659 |
| Min | 0.023 | 0.35 | 522 | 6252 | 0.005 | 0.21 | 119 | 1206 |
| As | As | |||||||
| Mean | <BDL 2 | <BDL | 0.969 | 6.40 | <BDL | <BDL | 0.335 | 3.93 |
| Max | <BDL | <BDL | 1.740 | 10.70 | 0.004 | <BDL | 0.734 | 9.60 |
| Min | <BDL | <BDL | 0.568 | 2.64 | <BDL | <BDL | 0.171 | 1.92 |
| Ba | Ba | |||||||
| Mean | 0.733 | 91.59 | 74.13 | 447.1 | 0.390 | 22.39 | 16.90 | 46.7 |
| Max | 1.430 | 143.00 | 137.43 | 1347.0 | 0.833 | 47.30 | 39.15 | 210.0 |
| Min | 0.270 | 37.40 | 31.41 | 63.9 | 0.125 | 7.89 | 4.44 | 9.8 |
| Be | Be | |||||||
| Mean | <BDL | <BDL | 0.315 | 0.89 | <BDL | <BDL | 0.107 | 0.37 |
| Max | <BDL | 0.002 | 0.433 | 1.11 | <BDL | 0.004 | 0.202 | 0.82 |
| Min | <BDL | <BDL | 0.189 | 0.56 | <BDL | <BDL | 0.053 | 0.13 |
| Ca | Ca | |||||||
| Mean | / | 10,457 | 38,110 | 39,469 | / | 7498 | 33,834 | 37,257 |
| Max | / | 13,628 | 76,460 | 78,479 | / | 12,313 | 64,672 | 86,503 |
| Min | / | 3686 | 11,498 | 12,026 | / | 1074 | 7019 | 8604 |
| Cd | Cd | |||||||
| Mean | <BDL | 0.063 | 0.213 | 0.43 | <BDL | 0.017 | 0.073 | 0.19 |
| Max | 0.001 | 0.118 | 0.310 | 0.59 | 0.001 | 0.048 | 0.163 | 0.35 |
| Min | <BDL | 0.042 | 0.139 | 0.26 | <BDL | <BDL | 0.041 | 0.10 |
| Co | Co | |||||||
| Mean | 0.001 | 0.018 | 3.75 | 9.47 | 0.002 | 0.006 | 1.36 | 4.33 |
| Max | 0.003 | 0.033 | 5.00 | 12.70 | 0.017 | 0.016 | 2.98 | 8.21 |
| Min | <BDL | 0.011 | 2.08 | 5.52 | <BDL | <BDL | 0.72 | 2.28 |
| Cr | Cr | |||||||
| Mean | <BDL | <BDL | 4.58 | 25.70 | <BDL | <BDL | 1.84 | 10.32 |
| Max | 0.001 | <BDL | 27.00 | 65.50 | 0.002 | <BDL | 14.61 | 29.30 |
| Min | <BDL | <BDL | 0.71 | 12.40 | <BDL | <BDL | 0.01 | 4.12 |
| Cu | Cu | |||||||
| Mean | 0.011 | 0.466 | 17.1 | 18.5 | 0.005 | 0.360 | 12.6 | 8.1 |
| Max | 0.015 | 0.552 | 28.3 | 26.2 | 0.011 | 1.420 | 22.9 | 14.9 |
| Min | 0.006 | 0.347 | 10.1 | 10.2 | <BDL | 0.188 | 3.6 | 2.9 |
| Fe | Fe | |||||||
| Mean | 0.190 | 2.16 | 2903 | 27,903 | 0.275 | 1.13 | 1267 | 15,019 |
| Max | 0.307 | 5.99 | 4208 | 39,145 | 1.090 | 3.41 | 2082 | 34,941 |
| Min | 0.067 | <BDL | 2091 | 20,314 | 0.040 | <BDL | 681 | 4092 |
| K | K | |||||||
| Mean | 2.25 | 78.8 | 122.2 | 723.5 | 0.76 | 21.3 | 44.8 | 269.0 |
| Max | 2.61 | 105.0 | 152.3 | 930.0 | 1.87 | 55.7 | 70.3 | 510.0 |
| Min | 1.66 | 65.2 | 88.1 | 482.0 | 0.27 | 9.6 | 14.8 | 77.9 |
| Li | Li | |||||||
| Mean | <BDL | 0.070 | 5.70 | 25.01 | <BDL | 0.027 | 4.91 | 14.27 |
| Max | <BDL | 0.094 | 10.42 | 29.40 | <BDL | 0.039 | 8.50 | 25.50 |
| Min | <BDL | 0.041 | 2.47 | 18.00 | <BDL | 0.017 | 1.37 | 3.93 |
| Fraction: <63 μm | Fraction: 63–2000 μm | |||||||
|---|---|---|---|---|---|---|---|---|
| [mg kg−1] | CaC 1 | AA | NA | BCR | CaC | AA | NA | BCR |
| Mg | Mg | |||||||
| Mean | 37.0 | 503 | 6468 | 9072 | 20.2 | 178 | 10,233 | 10,578 |
| Max | 73.8 | 891 | 10,152 | 11,502 | 56.5 | 355 | 23,943 | 27,520 |
| Min | 17.6 | 339 | 3240 | 4949 | 4.7 | 74 | 1123 | 1455 |
| Mn | Mn | |||||||
| Mean | 0.68 | 24.6 | 231 | 457 | 0.85 | 11.1 | 101 | 286 |
| Max | 1.23 | 54.2 | 423 | 881 | 2.72 | 22.0 | 321 | 946 |
| Min | 0.08 | 10.9 | 69 | 164 | 0.12 | 3.0 | 30 | 83 |
| Na | Na | |||||||
| Mean | 2.87 | 48.8 | 45.4 | 105.5 | 0.66 | 16.7 | 20.6 | 48.5 |
| Max | 5.64 | 87.7 | 73.5 | 159.0 | 1.16 | 26.0 | 34.4 | 69.6 |
| Min | 1.48 | 29.8 | 31.1 | 64.0 | 0.43 | 10.9 | 8.9 | 18.6 |
| Ni | Ni | |||||||
| Mean | 0.004 | 0.222 | 4.30 | 23.29 | 0.007 | 0.082 | 1.32 | 9.47 |
| Max | 0.010 | 0.330 | 6.00 | 31.50 | 0.035 | 0.125 | 2.33 | 18.00 |
| Min | 0.002 | 0.141 | 1.93 | 13.40 | 0.000 | 0.042 | 0.59 | 4.11 |
| P | P | |||||||
| Mean | 0.081 | 6.64 | 216.2 | 5817 | 0.031 | 2.12 | 88.8 | 2877 |
| Max | 0.137 | 13.00 | 260.8 | 6999 | 0.147 | 9.86 | 154.5 | 4635 |
| Min | 0.015 | 0.00 | 180.0 | 4414 | <BDL 2 | 0.00 | 45.4 | 1058 |
| Pb | Pb | |||||||
| Mean | <BDL | <BDL | 11.45 | 22.78 | <BDL | <BDL | 3.26 | 8.21 |
| Max | <BDL | <BDL | 15.65 | 31.80 | <BDL | <BDL | 5.42 | 20.80 |
| Min | <BDL | <BDL | 5.47 | 10.60 | <BDL | <BDL | 1.57 | 2.89 |
| S | S | |||||||
| Mean | 8.08 | 100.51 | 95.9 | 364 | 3.02 | 34.82 | 28.0 | 123 |
| Max | 18.40 | 230.00 | 187.8 | 600 | 15.60 | 163.00 | 112.5 | 656 |
| Min | 3.26 | 41.70 | 32.8 | 158 | 0.36 | 6.21 | 5.2 | 35 |
| Si | Si | |||||||
| Mean | 6.24 | 101.1 | 730 | 1585 | 2.11 | 17.7 | 245 | 739 |
| Max | 7.93 | 165.0 | 874 | 2115 | 4.32 | 57.9 | 458 | 1531 |
| Min | 4.43 | 54.3 | 566 | 1111 | 1.00 | 5.6 | 128 | 393 |
| Sr | Sr | |||||||
| Mean | 0.104 | 9.27 | 16.40 | 24.59 | 0.008 | 5.12 | 10.94 | 14.16 |
| Max | 0.253 | 14.40 | 27.15 | 37.20 | 0.073 | 10.20 | 18.49 | 31.80 |
| Min | 0.011 | 4.87 | 6.59 | 10.10 | <BDL | 1.29 | 2.81 | 4.35 |
| Ti | Ti | |||||||
| Mean | <BDL | <BDL | 5.06 | 76.06 | 0.004 | 0.013 | 2.89 | 53.85 |
| Max | 0.001 | 0.003 | 7.87 | 104.00 | 0.053 | 0.229 | 8.36 | 100.00 |
| Min | <BDL | <BDL | 3.42 | 49.30 | <BDL | <BDL | 0.82 | 27.40 |
| V | V | |||||||
| Mean | 0.001 | 0.041 | 2.13 | 19.21 | <BDL | 0.024 | 0.83 | 8.84 |
| Max | 0.003 | 0.104 | 3.13 | 25.00 | 0.002 | 0.073 | 1.51 | 14.60 |
| Min | <BDL | 0.018 | 1.20 | 11.90 | <BDL | 0.003 | 0.48 | 4.91 |
| Zn | Zn | |||||||
| Mean | 0.002 | 0.557 | 19.08 | 60.96 | 0.007 | 0.358 | 6.68 | 26.65 |
| Max | 0.008 | 0.904 | 28.07 | 85.35 | 0.091 | 1.000 | 12.86 | 56.26 |
| Min | <BDL | 0.238 | 13.02 | 37.49 | <BDL | 0.054 | 3.26 | 8.52 |
| <63 μm | 63–2000 μm | |||||
|---|---|---|---|---|---|---|
| Mean | Max | Min | Mean | Max | Min | |
| Zn | 60.95 | 85.30 | 37.50 | 26.65 | 56.30 | 8.52 |
| Al | 9.25 | 11.00 | 7.95 | 7.58 | 13.90 | 4.66 |
| As | 16.36 | 27.84 | 9.91 | 9.89 | 18.30 | 3.02 |
| Ba | 26.13 | 49.15 | 10.20 | 42.71 | 58.87 | 18.64 |
| Be | 35.63 | 44.21 | 29.66 | 32.29 | 52.87 | 17.42 |
| Ca | 96.84 | 108.76 | 86.80 | 93.41 | 114.40 | 70.37 |
| Cd | 49.44 | 59.33 | 40.39 | 39.29 | 67.70 | 17.62 |
| Co | 39.24 | 43.56 | 34.17 | 32.23 | 46.47 | 21.49 |
| Cu | 111.50 | 244.79 | 38.60 | 142.28 | 278.00 | 28.00 |
| Fe | 10.67 | 13.50 | 7.92 | 10.74 | 30.49 | 3.83 |
| K | 17.26 | 20.11 | 12.75 | 18.77 | 32.54 | 10.99 |
| Li | 23.77 | 41.84 | 9.00 | 37.60 | 68.23 | 9.79 |
| Mg | 70.79 | 88.26 | 59.82 | 100.37 | 219.08 | 66.61 |
| Mn | 50.12 | 56.79 | 41.89 | 39.63 | 62.43 | 22.70 |
| Na | 43.25 | 52.84 | 35.21 | 45.15 | 71.42 | 23.13 |
| Ni | 17.99 | 20.63 | 14.41 | 14.51 | 21.47 | 10.11 |
| Pb | 50.28 | 53.15 | 47.25 | 46.10 | 108.19 | 20.19 |
| S | 25.91 | 38.96 | 14.26 | 24.88 | 55.30 | 6.09 |
| Si | 46.74 | 52.29 | 38.31 | 34.73 | 57.13 | 17.86 |
| Sr | 65.79 | 76.73 | 44.91 | 79.09 | 109.38 | 58.15 |
| Ti | 6.74 | 10.16 | 4.45 | 5.46 | 13.13 | 1.62 |
| V | 10.93 | 13.72 | 8.58 | 9.80 | 15.57 | 4.83 |
| P | 3.76 | 4.32 | 2.68 | 3.26 | 4.81 | 2.08 |
| Cr | 11.22 | 41.22 | 3.60 | 11.87 | 49.85 | 0.07 |
| Pb | Ni | Cu | Cr | Cd | Zn | |
|---|---|---|---|---|---|---|
| 1 CaC | <BDL | 0.004 | 0.011 | <BDL | <BDL | 0.002 |
| AA | <BDL | 0.222 | 0.466 | <BDL | 0.063 | 0.557 |
| NA | 11.45 | 4.30 | 17.1 | 4.58 | 0.213 | 19.08 |
| BCR | 22.78 | 23.29 | 18.5 | 25.70 | 0.43 | 60.96 |
| 2 CaC | 6.83 | 0.13 | 0.15 | 4.4 3 | 0.7 3 | <BDL |
| 2 PTC | 29 | 17 | 106 | 11.0 | 4.0 | 8.2 |
| 4 CaC | <BDL-5974 3 | nd | 101–5589 3 | nd | 27–932 3 | 0.22–217 |
| E_AA | E_NA | E_BCR | |
|---|---|---|---|
| 1E_CaC | Ba **, K **, Mg **, Na **, P **, S **, Si **, Sr ** | Ba **, K **, Na **, P *, S **, Si **, Sr * | Ba **, Cu *, K **, Mn *, Na **, Si **, Sr ** |
| E_AA | Al *, Ba **, Cd **, Co *, Fe *, K **, Mn **, Na **, Ni **, S **, Si **, Sr * | Al *, Ba **, Cd **, K **, Li **, Na **, Ni **, S **, Si **, Sr *, Ti *, V * | |
| E_NA | Al **, As **, Ba **, Be **, Ca **, Cd **, Co **, Cr **, Fe **, K **, Mg **, Mn **, Na **, Ni **, P **, Pb **, S **, Si **, Sr **, Ti **, V **, Zn ** |
| Value of Eri | Risk Level | Probability (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Cu | Ni | Pb | Zn | ||
| Eri < 40 40 ≤ Eri < 80 80 ≤ Eri < 160 160 ≤ Eri < 320 Eri ≥ 320 | Low | 94.07 | 19.98 | 100 | 100 | 100 | 100 | 100 |
| Lower | 5.93 | 78.93 | 0 | 0 | 0 | 0 | 0 | |
| Median | 0 | 1.09 | 0 | 0 | 0 | 0 | 0 | |
| High | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Extremely high | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| HRI Value | Risk Level | Probability (%) |
|---|---|---|
| HRI < 150 | Low | 100 |
| 150 ≤ HRI < 300 | Lower | 0 |
| 300 ≤ Eri < 600 | Median | 0 |
| Eri ≥ 600 | High | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakan, S.; Frančišković-Bilinski, S.; Đorđević, D.; Popović, A.; Sakan, N.; Škrivanj, S.; Bilinski, H. Evaluation of Element Mobility in River Sediment Using Different Single Extraction Procedures and Assessment of Probabilistic Ecological Risk. Water 2021, 13, 1411. https://doi.org/10.3390/w13101411
Sakan S, Frančišković-Bilinski S, Đorđević D, Popović A, Sakan N, Škrivanj S, Bilinski H. Evaluation of Element Mobility in River Sediment Using Different Single Extraction Procedures and Assessment of Probabilistic Ecological Risk. Water. 2021; 13(10):1411. https://doi.org/10.3390/w13101411
Chicago/Turabian StyleSakan, Sanja, Stanislav Frančišković-Bilinski, Dragana Đorđević, Aleksandar Popović, Nenad Sakan, Sandra Škrivanj, and Halka Bilinski. 2021. "Evaluation of Element Mobility in River Sediment Using Different Single Extraction Procedures and Assessment of Probabilistic Ecological Risk" Water 13, no. 10: 1411. https://doi.org/10.3390/w13101411