Incidence of Watershed Land Use on the Consumption of Meso and Microplastics by Fish Communities in Uruguayan Lowland Streams
Abstract
:1. Introduction
2. Materials and Methods
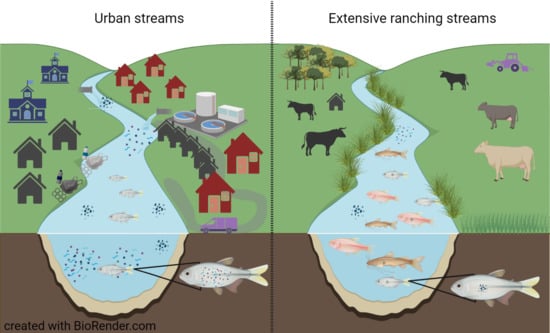
2.1. Study Sites
2.2. Environmental and Fish Communities Sampling
2.3. Plastics Consumption
2.4. Estimation of Plastics at Species and Community Level
3. Statistical Analysis
4. Results
4.1. Fish Communities
4.2. Plastic Debris
4.2.1. Community Level and Land Use
4.2.2. Trophic Level Group Analysis
4.2.3. Species and Individual Level
5. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wetzel, R.G.; Likens, G.E. Limnological Analyses, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Allan, D.J.; Castillo, M.M. Stream Ecology: Structure and Function of Running Waters, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Wagner, M.; Scherer, C.; Alvarez-Muñoz, D.; Brennholt, N.; Bourrain, X.; Buchinger, S.; Fries, E.; Grosbois, C.; Klasmeier, J.; Marti, T.; et al. Microplastics in freshwater ecosystems: What we know and what we need to know. Environ. Sci. Eur. 2014, 26, 12. [Google Scholar] [CrossRef] [Green Version]
- Pazos, R.S.; Maiztegui, T.; Colautti, D.C.; Paracampo, A.H. Microplastics in gut contents of coastal freshwater fish from Río de la Plata estuary. Mar. Pollut. Bull. 2017, 122, 85–90. [Google Scholar] [CrossRef]
- Horton, A.A.; Dixon, S.J. Microplastics: An introduction to environmental transport processes. WIREs Water. 2017, 5, e1268. [Google Scholar] [CrossRef] [Green Version]
- Sá, L.C.; Oliveira, M.; Ribeiro, F.; Lopes Rocha, T.; Futter, M.N. Studies of the effects of microplastics on aquatic organisms: What do we know and where should we focus our efforts in the future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [PubMed]
- Wright, S.L.; Ulke, J.; Font, A.; Chan, K.L.A.; Kelly, F.J. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environ. Int. 2019, 136, 105411. [Google Scholar] [CrossRef]
- Lozoya, J.P.; Teixeira de Mello, F.; Carrizo, D.; Weinstein, F.; Olivera, Y.; Cedrés, F.; Pereira, M.; Fossati, M. Plastic and microplastics on recreational beaches in Punta del Este (Uruguay): Unseen critical residents? Environ. Pollut. 2016, 218, 913–941. [Google Scholar] [CrossRef]
- Bellasi, A.; Binda, G.; Pozzi, A.; Galafassi, S.; Volta, P.; Bettinetti, R. Microplastic Contamination in Freshwater Environments: A Review, Focusing on Interactions with Sediments and Benthic Organisms. Environments 2020, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-) plastic particles. Environ. Chem. 2015, 12, 539–550. [Google Scholar] [CrossRef]
- Mason, S.A.; Daily, J.; Aleid, G.; Ricotta, R.; Smith, M.; Donnelly, K.; Knauff, R.; Edwards, W.; Hoffman, M.J. High levels of pelagic plastic pollution within the surface waters of Lakes Erie and Ontario. J. Great Lakes Res. 2020, 2, 277–288. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Smith, K.L. Plastics on the Sargasso Sea Surface. Science 1972, 175, 1240–1241. [Google Scholar] [CrossRef]
- Garcia, T.D.; Cardozo, A.L.P.; Quirino, B.A.; Yofukuji, K.Y.; Ganassin, M.; Dos Santos, N.; Fugi, R. Ingestion of microplastic by fish of different feeding habits in urbanized and non-urbanized streams in Southern Brazil. Water Air Soil Pollut. 2020, 231, 434. [Google Scholar] [CrossRef]
- Druis, K.; Coors, A. Microplastics in the aquatic and terrestrial environment: Sources (with a specific focus on personal care products), date and effects. Environ. Sci. Eur. 2016, 28, 1–25. [Google Scholar]
- World Economic Forum; Ellen MacArthur Foundation and McKinsey & Company. The New Plastics Economy—Rethinking the future of plastics. 2016. Available online: http://www.ellenmacarthurfoundation.org/publications (accessed on 4 February 2021).
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Pedà, C.; Caccamo, L.; Fossi, M.C.; Gai, F.; Andaloro, F.; Genovese, L.; Perdichizzi, A.; Romeo, T.; Marcchiolo, G. Intestinal alterations in European sea bass Dicentrarchuslabrax (Linnaeus, 1758) exposed to microplastics: Preliminary results. Environ. Pollut. 2016, 212, 251–256. [Google Scholar] [CrossRef]
- Wagner, M.; Lambert, S. Freshwater microplastics, emerging environmental contaminants? In The Handbook of Environmental Chemistry; Springer Open: Berlin/Heidelberg, Germany, 2018; Volume 58. [Google Scholar]
- Bergmann, M.; Gutow, L.; Klages, M. Marine Anthropogenic Litter; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Wang, W.; Ge, J.; Yu, X. Bioavailability and toxicity of microplastics to fish species: A review. Ecotoxicol. Environ. Saf. 2020, 189, 109913. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Ribeiro, A.; Hylland, K.; Guilhermino, L. Single and combined effects of microplastics and pyrene on juveniles (0+ group) of the common goby Pomatoschistusmicrops (Teleosteo, Gobiidae). Ecol. Indic. 2013, 34, 641–647. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef] [PubMed]
- Mazurais, D.; Ernande, B.; Quazuguel, P.; Severe, A.; Huelvan, C.; Madec, L.; Mouchel, O.; Soudant, P.; Robbens, J.; Huvet, A.; et al. Evaluation of the impact of polyethylene microbeads ingestion in European sea bass (Dicentrarchuslabrax) larvae. Mar. Environ. Res. 2015, 112, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Nobre, C.R.; Santana, M.F.M.; Maluf, A.; Cortez, F.S.; Cesar, A.; Pereira, C.D.S.; Turra, A. Assessment of microplastic toxicity to embryonic development of the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Mar. Pollut. Bull. 2015, 92, 99–104. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Rocher, V.; Saad, M.; Renault, N.; Tassin, B. Microplastic contamination in an urban area: A case study in Greater Paris. Environ. Chem. 2015, 12, 592–599. [Google Scholar] [CrossRef]
- Tosetto, L.; Williamson, J.E.; Brown, C. Trophic transfer of microplastics does not affect fish personality. Anim. Behav. 2017, 123, 159–167. [Google Scholar] [CrossRef]
- Fischer, E. Final Report: Distribution of Microplastics in Marine Species of Wadden Sea along the Coastline of Schleswig-Holstein; University of Hamburg: Hamburg, Germany, 2019. [Google Scholar]
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Lenzi, J.; Burgues, M.F.; Carrizo, D.; Machín, E.; Teixeira de Mello, F. Plastic ingestion by a generalist seabird on the coast of Uruguay. Mar. Pollut. Bull. 2016, 107, 71–76. [Google Scholar] [CrossRef]
- Burgues, M.F.; Lenzi, J.; Machín, E.; Genta, L.; Teixeira de Mello, F. Temporal Variation of Kelp Gull’s (Larusdominicanus) fiet on a coastal island of the Rio de la Plata estuary, Uruguay: Refuse as an alternative food source. Waterbirds 2020, 43, 65. [Google Scholar] [CrossRef]
- Azevedo-Santos, V.M.; Geslaine, R.L.; Gonçalves, P.; Andrade, M.; Lima, F.; Pelicice, F.M. Plastic ingestion by fish: A global assessment. Environ. Pollut. 2019, 255, 112994. [Google Scholar] [CrossRef]
- Flecker, A.S.; Taylor, B.W.; Bernhardt, J.; Hood, J.; Cornwell, W.K.; Cassatt, S.R.; Vanni, M.J.; Altman, N.S. Interactions between herbivorous fishes and limiting nutrients in a tropical stream ecosystem. Ecology 2002, 83, 1831–1844. [Google Scholar] [CrossRef]
- Vanni, M.J. Preface: When and where do fish have strong effects on stream ecosystem processes? Am. Fish. Soc. Symp. 2010, 73, 531–538. [Google Scholar]
- Villéger, S.; Brosse, S.; Mouchet, M.; Mouillot, D.; Vanni, M.J. Functional ecology of fish: Current approaches and future challenges. Aquat. Sci. 2017, 79, 783–801. [Google Scholar] [CrossRef]
- Silva, I.; Naya, D.; Teixeira de Mello, F.; D’Anatro, A.; Tesitore, G.; Clavijo, C.; Gonzáles-Bergonzoni, I. Fish vs. Aliens: Predatory fish regulate populations of Limnopernafortunei mitigating impacts on native macroinvertebrate communities. Hydrobiologia 2021, 848, 2281–2301. [Google Scholar] [CrossRef]
- Alvareda, E.; Lucas, C.; Paradiso, M.; Piperno, A.; Gamazo, P.; Erasun, V.; Russo, P.; Saracho, E.; Banega, R.; Sapriza, G.; et al. Water quality evaluation of two urban streams in Northwest Uruguay: Are national regulations for urban stream quality sufficient? Environ. Monit. Assess. 2020, 192, 661. [Google Scholar] [CrossRef]
- Valderrama, J.C. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Mar. Chem. 1981, 10, 109–122. [Google Scholar] [CrossRef]
- Teixeira de Mello, F.; Kristensen, E.A.; Meerhoff, M.; González- Bergonzoni, I.; Baattrup-Pedersen, A.; Iglesias, C.; Kristensen, P.B.; Mazzeo, N.; Jeppesen, E. Monitoring fish communities in wadeable lowland streams: Comparing the efficiency of electrofishing methods at contrasting fish assemblages. Environ. Monit. Assess. 2014, 186, 1665–1677. [Google Scholar] [CrossRef]
- Teixeira de Mello, F.; González-Bergonzoni, I.; Loureiro, M. Peces de Agua dulce del Uruguay; PPR-MGAP: Montevideo, Uruguay, 2011; p. 188. [Google Scholar]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [Green Version]
- GESAMP. Guidelines for the Monitoring and Assessment of Plastic Litter in the Ocean; Kershaw, P.J., Turra, A., Galgani, F., Eds.; IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP/ISA Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection; United Nations Office Nairobi (UNON): Nairobi, Kenya, 2019; Volume 99, p. 130. [Google Scholar]
- Chang, M. Reducing microplastics from facial exfoliating cleansers in wastewater through treatment versus consumer product decisions. Mar. Pollut. Bull. 2015, 101, 330–333. [Google Scholar] [CrossRef]
- Hidalgo-Ruiz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.; Lima, A.R.A.; Costa, M.F. Distribution sources and consequences of nutrients, persistent organic pollutants, metals and microplastics in South American estuaries. Sci. Total Environ. 2019, 651, 1199–1218. [Google Scholar] [CrossRef]
- Dehaut, A.; Cassone, A.L.; Frére, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Riviére, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, I.; Rodríguez Chialanza, M.; Faccio, R.; Carrizo, D.; Fornaro, L.; Pérez-Parada, A. Identification of microplastics in wastewater samples by means of polarized light optical microscopy. Environ. Sci. Pollut. Res. Int. 2019, 27, 7409–7419. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.; Welden, N.A.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods. 2016, 9, 1346. [Google Scholar]
- Hammer, Ø. PAST: Paleontological Statistics, Version 3.25; Reference manual; Natural History Museum, University of Oslo: Oslo, Norway, 2019. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Harting, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2020. Available online: http://florianhartig.github.io/DHARMa/ (accessed on 4 February 2021).
- Benejam, L.; Teixeira de Mello, F.; Meerhoff, M.; Loureiro, M.; Jeppesen, E.; Brucet, S. Assessing effects of change in land use on sixe-related variables of fish in subtropical streams. Can. Sci. Publ. 2015, 73, 547–556. [Google Scholar] [CrossRef]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kideyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Ŝtindlová, A.; Martínez, I.; Rapp, J.; Romero-Kutzner, V.; Samper, M.D.; Montoto, T.; Aguiar-González, B.; Paclard, T.; Gómez, M. Microplastic ingestion by Atlantic chub mackerel (Scombercolias) in the Canary Islands coast. Mar. Pollut. Bull. 2019, 139, 127–135. [Google Scholar] [CrossRef]
- Dos Santos, T.; Bastian, R.; Felden, J.; Rauber, A.M.; Reynalte-Tataje, D.A.; Teixeira de Mello, F. First record of microplastics in two freshwater fish species (Iheringhthyslabrosus and Astyanax lacustris) from the middle section of the Uruguay River, Brazil. Acta Limnol. Bras. 2020, 32. [Google Scholar] [CrossRef]
- Martí, E.; Martin, C.; Galli, M.; Echevarría, F.; Duarte, C.M.; Cózar, A. The colors of the ocean plastics. Environ. Sci. Technol. 2020, 54, 6594–6601. [Google Scholar] [CrossRef]
- Roch, S.; Friedrich, C.; Brinker, A. Uptake routes of microplastics in fishes: Practical and theoretical approaches to test existing theories. Sci. Rep. 2020, 10, 3896. [Google Scholar] [CrossRef]
- Andrade, M.C.; Winemiller, K.O.; Barbosa, P.S.; Fortunati, A.; Chelazzi, D.; Cincinelli, A.; Giarrizzo, T. First account of plastic pollution impacting freshwater fishes in the Amazon: Ingestion of plastic debris by piranhas and other serrasalmids with diverse feeding habits. Environ. Pollut. 2019, 244, 766–773. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Durántez Jiménez, P.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric transport and deposition of microplastics in a remote mountain catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Foley, C.J.; Feiner, Z.S.; Malinich, T.D.; Höök, T.O. A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci. Total Environ. 2018, 631–632, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Chernick, M.; Hinton, D.E.; Shi, H. Microplastics in small waterbodies and tadpoles from Yangtze River Delta, China. Environ. Sci. Technol. 2018, 52, 8885–8893. [Google Scholar] [CrossRef]
- Wen, X.; Du, C.; Xu, P.; Zeng, G.; Huang, D.; Yin, L.; Tin, Q.; Hu, L.; Wan, J.; Zhang, J.; et al. Microplastic pollution in surface sediments of urban water areas in Changsha, China: Abundance, composition, surface textures. Mar. Pollut. Bull. 2018, 136, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, B. Ingestion of microplastics by fish and it’s potential consequences from a physical perspective. Integr. Environ. Assess. Manag. 2017, 13, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 0116. [Google Scholar] [CrossRef]
- Mizraji, R.; Ahrendt, C.; Perez-Venegas, D.; Vargas, J.; Pulgar, J.; Aldana, M.; Ojeda, F.P.; Duarte, C.; Galbán-Malagón, C. Is the feeding type related with the content of microplastics in intertidal fish gut? Mar. Pollut. Bull. 2017, 1–2, 498–500. [Google Scholar] [CrossRef]
- Phillips, M.B.; Bonner, T.H. Occurrence and amount of microplastic ingested by fishes in watersheds of the Gulf of Mexico. Mar. Pollut. Bull. 2015, 100, 264–269. [Google Scholar] [CrossRef]
- Murphy, F.; Russell, M.; Ciaran, E.; Quinn, B. The uptake of macroplastic & microplastic by demersal & pelagic fish in the Northeast Atlantic around Scotland. Mar. Pollut. Bull. 2017, 122, 353–359. [Google Scholar]






| Extensive Ranching | Urban | |||||||
|---|---|---|---|---|---|---|---|---|
| Insaurral | Curupí | Las Niñas | Average | C. del Dragón | C. del Colorado | Las Piedras | Average | |
| Watershed (km2) | 14.42 | 16.32 | 4.421 | 11.7 ± 6.4 | 11.21 | 6.063 | 25.83 | 14.4 ± 10.3 |
| Order | 3 | 3 | 3 | - | 2 | 1 | 2 | - |
| Average width (m) | 1.67 | 3.03 | 1.97 | 2.2 ± 0.7 | 2.33 | 1.57 | 4.13 | 2.7 ± 1.3 |
| Average depth (m) | 0.19 | 0.45 | 0.34 | 0.32 ±0. 13 | 0.45 | 0.25 | 0.32 | 0.34 ± 0.10 |
| Land use (%) | ||||||||
| Urban area | 0.05 | 0 | 0 | 0.02 ± 0.03 | 13.17 | 29.03 | 34.52 | 25.6 ± 11.1 |
| Natural grassland | 67.66 | 15.76 | 68.7 | 50.7 ± 30.3 | 14.06 | 30.26 | 20.75 | 21.7 ± 8.1 |
| Riverine forest | 0 | 47.37 | 0 | 15.8 ± 27.3 | 4.65 | 0.02 | 1.22 | 2.0 ± 2.4 |
| Rainfed and irrigated crop | 0 | 0 | 0 | 0 | 62.64 | 23.42 | 37.75 | 41.3 ± 19.8 |
| Pastures | 32.1 | 8.81 | 31.12 | 24 ± 13.2 | 1.1 | 14.4 | 2.92 | 6.1 ± 7.2 |
| Afforestation | 0.19 | 28.07 | 0.18 | 9.5 ± 16.1 | 4.37 | 2.87 | 2.84 | 3.4 ± 0.9 |
| Physico-chemical parameters | ||||||||
| Dissolved oxygen | 8.78 | 8.22 | 11 | 9.3 ± 1.5 | 5.72 | 5.05 | 3.78 | 4.9 ± 1.0 |
| pH | 8.28 | 7.89 | 8.41 | 8.2 ± 0.3 | 7.38 | 7.43 | 7.66 | 7.5 ± 0.1 |
| Total Nitrogen (µg/L) | 497 | 275 | 673 | 481.7 ± 199 | 258 | 471 | 706 | 478.3 ± 224 |
| Total Phosphorus (µg/L) | 706 | 770 | 1170 | 882 ± 251 | 2689 | 1330 | 5862 | 3293.7 ± 2325 |
| Temperature (⁰C) | 24.4 | 25.46 | 23.7 | 24.5 ± 0.9 | 23.14 | 23.53 | 25.28 | 24 ± 11 |
| Extensive Ranching | Urban | |||||||
|---|---|---|---|---|---|---|---|---|
| Insaurral | Curupí | Las Niñas | Mean ± SD | C. del Dragón | C. del Colorado | Las Piedras | Mean ± SD | |
| Richness | 16 | 14 | 16 | 15.3 ± 1.2 | 17 | 11 | 5 | 11 ± 6.0 |
| Biomass (g/m2) | 10.84 | 7.11 | 7.97 | 8.6 ± 1.9 | 1.94 | 1.57 | 1.05 | 1.5 ± 0.5 |
| Density (ind/m2) | 11.11 | 2.89 | 8.95 | 7.7 ± 4.2 | 4.24 | 3.12 | 22.79 | 10.1 ± 11.1 |
| Relative abundance of species (%) | ||||||||
| Cnesterodon decemmaculatus | 12.85 | 30.18 | 19.83 | 21.0 ± 8.7 | 0.98 | 60 | 99.7 | 53.6 ± 49.7 |
| Characidium rachovii | 5.91 | 4.47 | 25 | 11.8 ± 11.5 | 1.47 | 3 | 0 | 1.5 ± 1.5 |
| Ectrepopterus uruguayensis | 7.97 | 0 | 20.65 | 9.5 ± 10.4 | 0 | 0 | 0 | 0.00 ± 0 |
| Astyanax sp. | 5.41 | 17.88 | 3.26 | 8.9 ± 7.9 | 10.78 | 10 | 0 | 6.9 ± 6.0 |
| Bryconamericus iheringii | 23.39 | 0 | 1.36 | 8.3 ± 13.1 | 21.57 | 1 | 0 | 7.5 ± 12.2 |
| Australoheros facetus | 12.08 | 8.37 | 0.54 | 7.0 ± 5.9 | 0 | 0 | 0 | 0.00 ± 0 |
| Cheirodon interruptus | 11.31 | 5.03 | 2.72 | 6.4 ± 4.5 | 10.78 | 6 | 0 | 5.6 ± 5.4 |
| Hyphessobrycon meridionalis | 0.76 | 2.23 | 8.97 | 4.0 ± 4.4 | 4.9 | 0 | 0 | 1.6 ± 2.8 |
| Gymnogeophagus terrapurpura | 5.14 | 3.91 | 2.45 | 3.8 ± 1.4 | 0.98 | 0 | 0.05 | 0.3 ± 0.6 |
| Heptapterus mustelinus | 6.43 | 4.47 | 0 | 3.6 ± 3.3 | 0.98 | 0 | 0 | 0.3 ± 0.6 |
| Gymnogeophagus mekinos | 1.03 | 7.82 | 1.36 | 3.4 ± 3.8 | 0 | 0 | 0 | 0.00 ± 0 |
| Crenicichla scottii | 3.34 | 5.58 | 0.82 | 3.3 ± 2.4 | 0 | 0 | 0 | 0.00 ± 0 |
| Steindachnerina biornata | 0 | 0 | 5.98 | 2.0 ± 3.5 | 0.98 | 1 | 0 | 0.7 ± 0.6 |
| Rineloricaria sp. | 2.06 | 2.23 | 0 | 1.4 ± 1.2 | 0 | 9 | 0 | 3.0 ± 5.2 |
| Australoheros scitulus | 0.26 | 0 | 3.53 | 1.3 ± 1.9 | 0 | 0 | 0 | 0.00 ± 0 |
| Charax stenopterus | 0 | 3.35 | 0.27 | 1.2 ± 1.9 | 0 | 0 | 0 | 0.00 ± 0 |
| Oligosarcus jenynsii | 0.51 | 1.12 | 1.63 | 1.1 ± 0.6 | 0.49 | 0 | 0 | 0.2 ± 0.3 |
| Synbranchus marmoratus | 0 | 1.68 | 0 | 0.6 ± 1.0 | 0.49 | 3 | 0 | 1.2 ± 1.6 |
| Hoplias argentinensis | 0 | 0 | 1.63 | 0.5 ± 0.9 | 0.49 | 0 | 0 | 0.2 ± 0.3 |
| Ancistrus taunayi | 0 | 1.12 | 0 | 0.4 ± 0.7 | 0 | 0 | 0 | 0.00 ± 0 |
| Corydoras paleatus | 0.77 | 0 | 0 | 0.3 ± 0.4 | 6.86 | 4 | 0.15 | 3.7 ± 3.4 |
| Hypostomus commersoni | 0 | 0.56 | 0 | 0.2 ± 0.3 | 0 | 0 | 0 | 0.00 ± 0 |
| Pseudocorynopoma doriae | 0.26 | 0 | 0 | 0.1 ± 0.2 | 4.9 | 0 | 0 | 1.6 ± 2.8 |
| Hisonotus nigricauda | 0.26 | 0 | 0 | 0.1 ± 0.2 | 1.47 | 0 | 0 | 0.5 ± 0.9 |
| Gymnotus omarorum | 0.26 | 0 | 0 | 0.1 ± 0.2 | 0 | 0 | 0 | 0.00 ± 0 |
| Phalloceros caudimaculatus | 0 | 0 | 0 | 0.0 ± 0 | 26.96 | 0 | 0 | 9.0 ± 15.6 |
| Otocinclus arnoldi | 0 | 0 | 0 | 0.0 ± 0 | 2.45 | 0 | 0.05 | 0.8 ± 1.4 |
| Rhamdia aff. quelen | 0 | 0 | 0 | 0.0 ± 0 | 0.49 | 2 | 0 | 0.8 ± 1.0 |
| Jenynsia lineata | 0 | 0 | 0 | 0.0 ± 0 | 0 | 1 | 0.05 | 0.4 ± 0.6 |
| Streams | items.m−2 Estymate by Biomass | items.m−2 Estymate by Density |
|---|---|---|
| Insaurral | 2.4 | 12.7 |
| Curupí | 1.2 | 3.1 |
| Las Niñas | 1.2 | 5.8 |
| Cañada del Dragón | 1.6 | 5.7 |
| Cañada del Colorado | 1.9 | 7.4 |
| Las Piedras | 3.2 | 24.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, C.; Lozoya, J.P.; Tesitore, G.; Goyenola, G.; Teixeira-de-Mello, F. Incidence of Watershed Land Use on the Consumption of Meso and Microplastics by Fish Communities in Uruguayan Lowland Streams. Water 2021, 13, 1575. https://doi.org/10.3390/w13111575
Vidal C, Lozoya JP, Tesitore G, Goyenola G, Teixeira-de-Mello F. Incidence of Watershed Land Use on the Consumption of Meso and Microplastics by Fish Communities in Uruguayan Lowland Streams. Water. 2021; 13(11):1575. https://doi.org/10.3390/w13111575
Chicago/Turabian StyleVidal, Camila, Juan Pablo Lozoya, Giancarlo Tesitore, Guillermo Goyenola, and Franco Teixeira-de-Mello. 2021. "Incidence of Watershed Land Use on the Consumption of Meso and Microplastics by Fish Communities in Uruguayan Lowland Streams" Water 13, no. 11: 1575. https://doi.org/10.3390/w13111575