Stream Hydrochemical Response to Flood Events in a Multi-Lithological Karstic Catchment from the Pyrenees Mountains (SW France)
Abstract
:1. Introduction
2. Materials and Methods
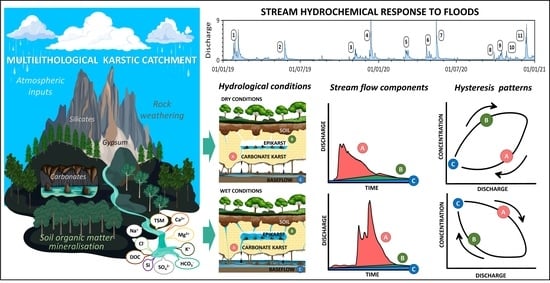
2.1. Study Area
2.2. Sampling and Analysis
Hydrochemical Measurements
2.3. Data Treatment
2.3.1. Hydrograph Separation Method
2.3.2. Analyses of C-Q Relationships
2.3.3. Hysteresis Analyses
2.3.4. Method of Flux Calculation
3. Results
3.1. Hydrochemical Survey during the Period 2019–2020
3.2. Hydrochemical Features of Flood Events
3.2.1. Flood Hydrological Characteristics
3.2.2. C-Q Relationships
3.3. Specific Patterns of Representative Flood Events
3.3.1. C-Q Patterns for Representative Floods
3.3.2. Typology of Flood Events
3.3.3. Hydrochemical Patterns and Streamflow Components during Floods 2 and 11
4. Discussion
4.1. Sources and Major Processes Controlling Streamwater Chemistry
4.2. Contribution of the Different Reservoirs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Baget Catchment |
| C | Concentration of Dissolved Elements |
| Cb | Concentration when the Discharge and Flux were Minimal (b = 0) |
| Cm | Mean Concentration of Each Dissolved Element |
| CV | Coefficient of Variation |
| Cond | Specific Conductivity |
| DCR | Discharge Change Rate |
| DO | Dissolved Oxygen |
| DOC | Dissolved Organic Carbon |
| eFE | End of Flood Event |
| K-CZ | Karstic Critical Zone |
| NICB | Net Inorganic Charge Balances |
| P | Rainfall |
| Q | Discharge |
| Qs | Specific discharge |
| pFE | Pre-Flood Event |
| ReP-Karst | Karst Dominated Recession Period |
| ReP-nKarst | Other Lithologies Dominated Recession Period |
| RiP | Rising Period |
| TDS | Total Dissolved Solids |
| Water T° | Water Temperature |
Appendix A
Appendix B
| Date | Ca2+ | Mg2+ | Na+ | K+ | SO42− | Cl− | NO3− |
|---|---|---|---|---|---|---|---|
| 10 October 2019 | 15.3 | 5.2 | 36.4 | 4.0 | 8.1 | 34.7 | 12.2 |
| 25 October 2019 | 11.0 | 1.6 | 6.0 | 3.3 | 3.4 | 6.0 | 5.8 |
| 22 November 2019 | 16.2 | 4.9 | 19.8 | 5.7 | 0.6 | 2.6 | 0.1 |
| 19 December 2019 | 7.1 | 4.1 | 32.8 | 4.5 | 2.3 | 42.9 | 1.3 |
| 23 January 2020 | 4.8 | 3.4 | 29.4 | 5.8 | 3.7 | 32.5 | 3.4 |
| 5 March 2020 | 5.7 | 4.3 | 43.2 | 4.2 | 6.0 | 42.9 | 2.7 |
| 23 April 2020 | 5.9 | 3.9 | 33.8 | 3.5 | 5.2 | 36.3 | 7.6 |
| 14 May 2020 | 9.8 | 1.7 | 7.5 | 7.4 | 5.0 | 9.1 | 7.0 |
| 28 May 2020 | 3.4 | 0.6 | 0.4 | 2.4 | 0.9 | 1.7 | 3.0 |
| 25 June 2020 | 18.5 | 5.0 | 15.3 | 5.9 | 7.5 | 19.4 | 17.8 |
| 24 September 2020 | 27.4 | 4.8 | 22.2 | 7.3 | 5.5 | 32.9 | 8.3 |
| 8 October 2020 | 4.3 | 4.4 | 30.2 | 3.5 | 1.6 | 23.9 | 1.0 |
| 22 October 2020 | 2.0 | 1.9 | 10.7 | 3.3 | 1.9 | 10.7 | 4.6 |
| 5 November 2020 | 25.6 | 6.5 | 10.6 | 21.9 | 2.3 | 23.2 | 4.3 |
| 17 December 2020 | 10.0 | 4.2 | 19.9 | 0.0 | 0.1 | 18.1 | 2.3 |
References
- Brantley, S.L.; Goldhaber, M.B.; Ragnarsdottir, K.V. Crossing Disciplines and Scales to Understand the Critical Zone. Elements 2007, 3, 307–314. [Google Scholar] [CrossRef]
- Council, N.R. Basic Research Opportunities in Earth Science; The National Academies Press: Washington, WA, USA, 2001; ISBN 978-0-309-07133-8. [Google Scholar]
- Richardson, J.B. Critical Zone. In Encyclopedia of Geochemistry. Encyclopedia of Earth Sciences; White, W.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–5. ISBN 978-3-319-39193-9. [Google Scholar]
- Bakalowicz, M.E. Encyclopedia of Caves; Academic Press: Cambridge, MA, USA, 2012; pp. 284–288. [Google Scholar]
- Bakalowicz, M. The Epikarst, the Skin of Karst. Karst Waters Inst. Spec. Publ. 9 Epikarst 2003, 9, 16–22. [Google Scholar]
- Bakalowicz, M. Karst groundwater: A challenge for new resources. Hydrogeol. J. 2005, 13, 148–160. [Google Scholar] [CrossRef]
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allègre, C. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Suchet, P.A.; Probst, J.-L.; Ludwig, W. Worldwide distribution of continental rock lithology: Implications for the atmospheric/soil CO2 uptake by continental weathering and alkalinity river transport to the oceans. Glob. Biogeochem. Cycles 2003, 17, 1038. [Google Scholar] [CrossRef] [Green Version]
- Messerli, B.; Viviroli, D.; Weingartner, R. Mountains of the world: Vulnerable water towers for the 21st century. Ambio 2004, 33, 29–34. [Google Scholar] [CrossRef]
- Lopez-Moreno, I.; Beniston, M.; García-Ruiz, J. Environmental change and water management in the Pyrenees: Facts and future perspectives for Mediterranean mountains. Glob. Planet. Chang. 2008, 61, 300–312. [Google Scholar] [CrossRef] [Green Version]
- Li, S.-L.; Xu, S.; Wang, T.-J.; Yue, F.-J.; Peng, T.; Zhong, J.; Wang, L.-C.; Chen, J.-A.; Wang, S.-J.; Chen, X.; et al. Effects of agricultural activities coupled with karst structures on riverine biogeochemical cycles and environmental quality in the karst region. Agric. Ecosyst. Environ. 2020, 303, 107120. [Google Scholar] [CrossRef]
- Tipper, E.; Bickle, M.J.; Galy, A.; West, A.J.; Pomiès, C.; Chapman, H.J. The short term climatic sensitivity of carbonate and silicate weathering fluxes: Insight from seasonal variations in river chemistry. Geochim. Cosmochim. Acta 2006, 70, 2737–2754. [Google Scholar] [CrossRef]
- Raymond, P.A.; Oh, N.-H.; Turner, R.E.; Broussard, W. Anthropogenically enhanced fluxes of water and carbon from the Mississippi River. Nat. Cell Biol. 2008, 451, 449–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakalowicz, M. Contribution de la Géochimie des Eaux a la Connaissance de l’aquifère Karstique et de la Karstification. Ph.D. Thesis, University Pierre et Marie Curie, Paris, France, 1979. [Google Scholar]
- Huang, X.; Fang, N.; Zhu, T.; Wang, L.; Shi, Z.; Hua, L. Hydrological response of a large-scale mountainous watershed to rainstorm spatial patterns and reforestation in subtropical China. Sci. Total Environ. 2018, 645, 1083–1093. [Google Scholar] [CrossRef]
- Yue, F.-J.; Waldron, S.; Li, S.-L.; Wang, Z.-J.; Zeng, J.; Xu, S.; Zhang, Z.-C.; Oliver, D. Land use interacts with changes in catchment hydrology to generate chronic nitrate pollution in karst waters and strong seasonality in excess nitrate export. Sci. Total Environ. 2019, 696, 134062. [Google Scholar] [CrossRef]
- Ulloa-Cedamanos, F.; Probst, J.-L.; Binet, S.; Camboulive, T.; Payre-Suc, V.; Pautot, C.; Bakalowicz, M.; Beranger, S.; Probst, A. A Forty-Year Karstic Critical Zone Survey (Baget Catchment, Pyrenees-France): Lithologic and Hydroclimatic Controls on Seasonal and Inter-Annual Variations of Stream Water Chemical Composition, pCO2, and Carbonate Equilibrium. Water 2020, 12, 1227. [Google Scholar] [CrossRef]
- Mcdonnell, J.; Bonell, M.; Stewart, M.K.; Pearce, A.J. Deuterium variations in storm rainfall: Implications for stream hydrograph separation. Water Resour. Res. 1990, 26, 455–458. [Google Scholar] [CrossRef]
- Laudon, H.; Slaymaker, O. Hydrograph separation using stable isotopes, silica and electrical conductivity: An alpine example. J. Hydrol. 1997, 201, 82–101. [Google Scholar] [CrossRef]
- Ladouche, B.; Probst, A.; Viville, D.; Idir, S.; Baqué, D.; Loubet, M.; Probst, J.-L.; Bariac, T. Hydrograph separation using isotopic, chemical and hydrological approaches (Strengbach catchment, France). J. Hydrol. 2001, 242, 255–274. [Google Scholar] [CrossRef]
- Pinder, G.F.; Jones, J.F. Determination of the ground-water component of peak discharge from the chemistry of total runoff. Water Resour. Res. 1969, 5, 438–445. [Google Scholar] [CrossRef]
- Sklash, M.G.; Farvolden, R.N. The Role Of Groundwater In Storm Runoff. Contemp. Hydrogeol. Georg. Burke Maxey Meml. Vol. 1979, 43, 45–65. [Google Scholar] [CrossRef]
- Maulé, C.P.; Stein, J. Hydrologic Flow Path Definition and Partitioning of Spring Meltwater. Water Resour. Res. 1990, 26, 2959–2970. [Google Scholar] [CrossRef]
- Probst, J.L. Hydrologie du bassin de la Garonne. Modèle de mélanges: Bilan de l’érosion: Exportation des phosphates et des nitrates. Ph.D. Thesis, University of Toulouse, Toulouse, France, 1983. [Google Scholar]
- Probst, J.; Tardy, Y. Fluctuations hydroclimatiques du Bassin d ’ Aquitaine au cours des 70 dernieres annees. Rev. Geol. Dyn. Geogr. Phys. 1985, 26, 59–76. [Google Scholar]
- Probst, J.; Bazerbachi, A. Transports en solution et en suspension par la Garonne supérieure. Solute and particulate transports by the upstream part of the Garonne river. Sci. Geol. Bull. 1986, 39, 79–98. [Google Scholar] [CrossRef]
- Evans, C.; Davies, T.D. Causes of concentration/discharge hysteresis and its potential as a tool for analysis of episode hydrochemistry. Water Resour. Res. 1998, 34, 129–137. [Google Scholar] [CrossRef]
- Darwiche-Criado, N.; Comín, F.A.; Sorando, R.; Sánchez-Pérez, J.M. Seasonal variability of NO3− mobilization during flood events in a Mediterranean catchment: The influence of intensive agricultural irrigation. Agric. Ecosyst. Environ. 2015, 200, 208–218. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Li, S.-L.; Waldron, S.; Yue, F.-J.; Wang, Z.-J.; Zhong, J.; Ding, H.; Liu, C.-Q. High-frequency monitoring reveals how hydrochemistry and dissolved carbon respond to rainstorms at a karstic critical zone, Southwestern China. Sci. Total. Environ. 2020, 714, 136833. [Google Scholar] [CrossRef]
- Musolff, A.; Schmidt, C.; Selle, B.; Fleckenstein, J.H. Catchment controls on solute export. Adv. Water Resour. 2015, 86, 133–146. [Google Scholar] [CrossRef]
- Kirchner, J.W.; Neal, C. Universal fractal scaling in stream chemistry and its implications for solute transport and water quality trend detection. Proc. Natl. Acad. Sci. USA 2013, 110, 12213–12218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Geer, F.C.; Kronvang, B.; Broers, H.P. High-resolution monitoring of nutrients in groundwater and surface waters: Process understanding, quantification of loads and concentrations, and management applications. Hydrol. Earth Syst. Sci. 2016, 20, 3619–3629. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Liu, Z.; Goldscheider, N.; Frank, S.; Goeppert, N.; Kaufmann, G.; Zeng, C.; Zeng, Q.; Sun, H. Comparisons on the effects of temperature, runoff, and land-cover on carbonate weathering in different karst catchments: Insights into the future global carbon cycle. Hydrogeol. J. 2021, 29, 331–345. [Google Scholar] [CrossRef]
- Nannoni, A.; Vigna, B.; Fiorucci, A.; Antonellini, M.; De Waele, J. Effects of an extreme flood event on an alpine karst system. J. Hydrol. 2020, 590, 125493. [Google Scholar] [CrossRef]
- Zhong, J.; Li, S.-L.; Tao, F.; Yue, F.-J.; Liu, C.-Q. Sensitivity of chemical weathering and dissolved carbon dynamics to hydrological conditions in a typical karst river. Sci. Rep. 2017, 7, srep42944. [Google Scholar] [CrossRef] [Green Version]
- Mangin, A. Contribution à l’étude Hydrodynamique des Aquifères Karstiques (Ann. Spéléo., 1974 29: 283-332; 1974 29: 495-601; 1975 30: 21-124;). Ph.D. Thesis, Université de Dijon, Dijon, France, 1975. [Google Scholar]
- Labat, D.; Ababou, R.; Mangin, A. Rainfall–runoff relations for karstic springs. Part I: Convolution and spectral analyses. J. Hydrol. 2000, 238, 123–148. [Google Scholar] [CrossRef]
- Debroas, E. Géologie du bassin versant du Baget (zone nord-pyrénéenne, Ariège, France): Nouvelles observations et conséquences. Strata 2009, 46, 1–93. [Google Scholar]
- Ulloa-Cedamanos, F.; Probst, A.; Moussa, I.; Probst, J. Chemical weathering and CO2 consumption in a multi-lithological karstic critical zone: Long term hydrochemical trends and isotopic survey. Chem. Geol. 2021. (under review). [Google Scholar]
- Jourde, H.; Massei, N.; Mazzilli, N.; Binet, S.; Batiot-Guilhe, C.; Labat, D.; Steinmann, M.; Bailly-Comte, V.; Seidel, J.; Arfib, B.; et al. SNO KARST: A French Network of Observatories for the Multidisciplinary Study of Critical Zone Processes in Karst Watersheds and Aquifers. Vadose Zone J. 2018, 17, 180094. [Google Scholar] [CrossRef] [Green Version]
- Gaillardet, J.; Braud, I.; Hankard, F.; Anquetin, S.; Bour, O.; Dorfliger, N.; De Dreuzy, J.; Galle, S.; Galy, C.; Gogo, S.; et al. OZCAR: The French Network of Critical Zone Observatories. Vadose Zone J. 2018, 17, 180067. [Google Scholar] [CrossRef] [Green Version]
- Parr, T.W.; Ferretti, M.; Simpson, I.C.; Forsius, M.; Kovács-Láng, E. Towards a long-term integrated monitoring programme in Europe: Network design in theory and practice. Environ. Monit. Assess. 2002, 78, 253–290. [Google Scholar] [CrossRef]
- Mirtl, M.; Borer, E.T.; Djukic, I.; Forsius, M.; Haubold, H.; Hugo, W.; Jourdan, J.; Lindenmayer, D.B.; McDowell, W.; Muraoka, H.; et al. Genesis, goals and achievements of Long-Term Ecological Research at the global scale: A critical review of ILTER and future directions. Sci. Total Environ. 2018, 626, 1439–1462. [Google Scholar] [CrossRef]
- Binet, S.; Probst, J.; Batiot, C.; Seidel, J.; Emblanch, C.; Peyraube, N.; Charlier, J.-B.; Bakalowicz, M.; Probst, A. Global warming and acid atmospheric deposition impacts on carbonate dissolution and CO2 fluxes in French karst hydrosystems: Evidence from hydrochemical monitoring in recent decades. Geochim. Cosmochim. Acta 2020, 270, 184–200. [Google Scholar] [CrossRef]
- ADES Database Point d’eau BSS002MAYC (10734X0010/HY) BAGET. Available online: https://ades.eaufrance.fr/Fiche/PtEau?Code=10734X0010/HY (accessed on 3 November 2020).
- Banque Hydro O0485110 Le Lachein à Balaguères [Baget-Las Hountas]. Available online: http://www.hydro.eaufrance.fr/ (accessed on 18 June 2021).
- Maillet, E. Essais d’Hydraulique Souterraine et Fluviale; Librairie Scientifique A. Herman: Paris, France, 1905. [Google Scholar]
- Dewandel, B.; Lachassagne, P.; Bakalowicz, M.; Weng, P.; Al-Malki, A. Evaluation of aquifer thickness by analysing recession hydrographs. Application to the Oman ophiolite hard-rock aquifer. J. Hydrol. 2003, 274, 248–269. [Google Scholar] [CrossRef]
- Probst, J. Nitrogen and phosphorus exportation in the Garonne Basin (France). J. Hydrol. 1985, 76, 281–305. [Google Scholar] [CrossRef]
- LucProbst, J.-L. Dissolved and suspended matter transported by the Girou River (France): Mechanical and chemical erosion rates in a calcareous molasse basin. Hydrol. Sci. J. 1986, 31, 61–79. [Google Scholar] [CrossRef] [Green Version]
- Godsey, S.E.; Kirchner, J.; Clow, D.W. Concentration-discharge relationships reflect chemostatic characteristics of US catchments. Hydrol. Process. 2009, 23, 1844–1864. [Google Scholar] [CrossRef]
- Lloyd, C.; Freer, J.; Johnes, P.; Collins, A. Using hysteresis analysis of high-resolution water quality monitoring data, including uncertainty, to infer controls on nutrient and sediment transfer in catchments. Sci. Total Environ. 2016, 543, 388–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butturini, A.; Alvarez, M.; Bernal, S.; Vazquez, E.; Sabater, F. Diversity and temporal sequences of forms of DOC and NO3-discharge responses in an intermittent stream: Predictable or random succession? J. Geophys. Res. Space Phys. 2008, 113, 1–10. [Google Scholar] [CrossRef]
- Lloyd, C.; Freer, J.E.; Johnes, P.J.; Collins, A. Technical Note: Testing an improved index for analysing storm discharge–concentration hysteresis. Hydrol. Earth Syst. Sci. 2016, 20, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Lawler, D.; Petts, G.; Foster, I.; Harper, S. Turbidity dynamics during spring storm events in an urban headwater river system: The Upper Tame, West Midlands, UK. Sci. Total Environ. 2006, 360, 109–126. [Google Scholar] [CrossRef]
- Meybeck, M. 5.08—Global Occurrence of Major Elements in Rivers; Holland, H.D., Turekian, K.T., Eds.; Treatise on Geochemistry, Pergamon Press: Oxford, UK, 2003; Volume 5, pp. 207–223. ISBN 9780080437514. [Google Scholar]
- Karim, A.; Veizer, J. Weathering processes in the Indus River Basin: Implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chem. Geol. 2000, 170, 153–177. [Google Scholar] [CrossRef]
- Dalai, T.; Krishnaswami, S.; Sarin, M. Major ion chemistry in the headwaters of the Yamuna river system. Geochim. Cosmochim. Acta 2002, 66, 3397–3416. [Google Scholar] [CrossRef]
- Millot, R.; Gaillardet, J.; Dupré, B.; Allègre, C.J. Northern latitude chemical weathering rates: Clues from the Mackenzie River Basin, Canada. Geochim. Cosmochim. Acta 2003, 67, 1305–1329. [Google Scholar] [CrossRef]
- Donnini, M.; Frondini, F.; Probst, J.-L.; Probst, A.; Cardellini, C.; Marchesini, I.; Guzzetti, F. Chemical weathering and consumption of atmospheric carbon dioxide in the Alpine region. Glob. Planet. Chang. 2016, 136, 65–81. [Google Scholar] [CrossRef] [Green Version]
- Calmels, D.; Gaillardet, J.; François, L. Sensitivity of carbonate weathering to soil CO2 production by biological activity along a temperate climate transect. Chem. Geol. 2014, 390, 74–86. [Google Scholar] [CrossRef]
- Lechuga-Crespo, J.; Sánchez-Pérez, J.; Sauvage, S.; Hartmann, J.; Suchet, P.A.; Probst, J.; Ruiz-Romera, E. A model for evaluating continental chemical weathering from riverine transports of dissolved major elements at a global scale. Glob. Planet. Chang. 2020, 192, 103226. [Google Scholar] [CrossRef]
- Qin, C.; Ding, H.; Li, S.-L.; Yue, F.-J.; Wang, Z.-J.; Zeng, J. Hydrogeochemical Dynamics and Response of Karst Catchment to Rainstorms in a Critical Zone Observatory (CZO), Southwest China. Front. Water 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Torres, M.A.; West, A.J.; Clark, K.E. Geomorphic regime modulates hydrologic control of chemical weathering in the Andes–Amazon. Geochim. Cosmochim. Acta 2015, 166, 105–128. [Google Scholar] [CrossRef] [Green Version]
- Clow, D.W.; Mast, M.A. Mechanisms for chemostatic behavior in catchments: Implications for CO2 consumption by mineral weathering. Chem. Geol. 2010, 269, 40–51. [Google Scholar] [CrossRef]
- Boy, J.; Valarezo, C.; Wilcke, W. Water flow paths in soil control element exports in an Andean tropical montane forest. Eur. J. Soil Sci. 2008, 59, 1209–1227. [Google Scholar] [CrossRef]
- Probst, A.; Viville, D.; Fritz, B.; Ambroise, B.; Dambrine, E. Hydrochemical budgets of a small forested granitic catchment exposed to acid deposition: The strengbach catchment case study (Vosges massif, France). Water Air Soil Pollut. 1992, 62, 337–347. [Google Scholar] [CrossRef]
- Pierret, M.-C.; Viville, D.; Dambrine, E.; Cotel, S.; Probst, A. Twenty-five year record of chemicals in open field precipitation and throughfall from a medium-altitude forest catchment (Strengbach-NE France): An obvious response to atmospheric pollution trends. Atmos. Environ. 2019, 202, 296–314. [Google Scholar] [CrossRef] [Green Version]
- Klimchouk, A.B. Towards defining, delimiting and classifying epikarst: Its origin, processes and variants of geomorphic evolution. Speleogenes Evol. Karst. Aquifers 2004, 2, 1–13. [Google Scholar]
- Zou, S.; Deng, Z.; Zhu, Y.; Liang, B.; Xia, R.; Tang, J. Hydrologic Features and Eco-environmental Classification of Epikarst Springs in Luota, West of Hunan, China. Earth Sci. Front. 2008, 15, 190–197. [Google Scholar] [CrossRef]
- Aquilina, L.; Ladouche, B.; Dörfliger, N. Water storage and transfer in the epikarst of karstic systems during high flow periods. J. Hydrol. 2006, 327, 472–485. [Google Scholar] [CrossRef]
- Trček, B. How can the epikarst zone influence the karst aquifer hydraulic behaviour? Environ. Earth Sci. 2006, 51, 761–765. [Google Scholar] [CrossRef]
- Walling, D.; Foster, I. Variations in the natural chemical concentration of river water during flood flows, and the lag effect: Some further comments. J. Hydrol. 1975, 26, 237–244. [Google Scholar] [CrossRef]
- Martínez-Santos, M.; Antiguedad, I.; Ruiz-Romera, E. Hydrochemical variability during flood events within a small forested catchment in Basque Country (Northern Spain). Hydrol. Process. 2013, 28, 5367–5381. [Google Scholar] [CrossRef]








| Parameter | Maximum | Minimum | Mean | DWM | Median | Std. Dev. | CV |
|---|---|---|---|---|---|---|---|
| Q | 8575.0 | 95.0 | 1046.7 | 525.8 (a) | 645.6 | 1077.3 | 1.03 |
| Water T° | 10.6 | 9.6 | 10.1 | 10.1 | 10.1 | 0.2 | 0.02 |
| Cond | 375.7 | 271.8 | 329.1 | 324.0 | 329.1 | 19.5 | 0.06 |
| pH | 8.5 | 7.3 | 7.9 | 7.8 | 7.8 | 0.2 | 0.02 |
| Ca2+ | 3542.9 | 1942.0 | 3081.6 | 3080.1 | 3120.6 | 203.0 | 0.07 |
| Mg2+ | 512.2 | 244.0 | 350.5 | 336.8 | 348.3 | 46.9 | 0.13 |
| Na+ | 67.1 | 22.6 | 40.4 | 39.8 | 41.9 | 6.9 | 0.17 |
| K+ | 31.6 | 5.7 | 13.3 | 13.6 | 12.4 | 4.2 | 0.32 |
| HCO3− | 4218.8 | 2094.2 | 3196.4 | 3206.7 | 3219.0 | 294.7 | 0.09 |
| SO42− | 658.4 | 0.1 | 197.3 | 149.1 | 171.0 | 106.5 | 0.54 |
| Cl− | 76.6 | 0.4 | 43.0 | 43.7 | 43.9 | 9.7 | 0.22 |
| NO3− | 64.7 | 0.0 | 29.0 | 28.4 | 28.8 | 8.6 | 0.30 |
| H4SiO4 | 60.3 | 30.4 | 44.4 | 42.8 | 43.4 | 5.6 | 0.13 |
| TDS (b) | 357.9 | 210.5 | 285.5 | 283.4 | 286.7 | 21.3 | 0.07 |
| DOC | 8.0 | 0.4 | 1.1 | 1.3 | 1.1 | 0.5 | 0.48 |
| Flood | Time before | Qmean-24h | Time Start Q | Start Q | Peak Q | End Q | Duration | Total Qs |
|---|---|---|---|---|---|---|---|---|
| 1 | 36.4 | 0.39 | 25 January 2019 12:00 | 0.31 | 6.96 | 0.70 | 18.47 | 250.0 |
| 2 | 99.5 | 0.24 | 24 May 2019 11:40 | 0.23 | 4.42 | 0.36 | 10.39 | 92.5 |
| 3 | 150.7 | 0.05 | 31 October 2019 13:40 | 0.05 | 2.60 | 0.46 | 14.70 | 76.3 |
| 4 | 25.3 | 0.24 | 9 December 2019 14:10 | 0.23 | 9.35 | 0.37 | 21.72 | 207.0 |
| 5 | 59.7 | 0.19 | 28 February 2020 00:15 | 0.20 | 2.35 | 0.36 | 21.12 | 133.6 |
| 6 | 31.9 | 0.17 | 20 April 2020 00:15 | 0.17 | 3.45 | 0.38 | 17.70 | 115.6 |
| 7 | 2.3 | 0.32 | 9 May 2020 00:30 | 0.30 | 9.24 | 0.48 | 14.87 | 163.3 |
| 8 | 125.4 | 0.12 | 25 September 2020 07:10 | 0.12 | 1.92 | 0.29 | 5.14 | 19.1 |
| 9 | 11.0 | 0.31 | 10 October 2020 10:10 | 0.28 | 2.29 | 0.35 | 9.92 | 63.7 |
| 10 | 4.6 | 0.23 | 23 October 2020 21:30 | 0.22 | 2.13 | 0.33 | 9.69 | 44.6 |
| 11 | 34.6 | 0.23 | 6 December 2020 04:10 | 0.23 | 8.25 | 0.43 | 14.90 | 165.3 |
| Initial dry Hydrological Conditions (a) | Initial Wet Hydrological Conditions (a) | |||||
|---|---|---|---|---|---|---|
| Qmax ≈ 2.3 m3·s−1 | Qmax ≈ 4.5 m3·s−1 | Qmax ≈ 2.1 m3·s−1 | Qmax ≈ 9.4 m3·s−1 | Qmax ≈ 8.3 m3·s−1 | ||
| Flood event number | 9 | 2 | 10 | 4 | 11 | |
| Number of peaks | 3 | 2 | 2 | 3 | 3 | |
| Shape of the flood hydrograph |  |  |  |  |  | |
| Hysteresis loops | Ca2+ HCO3− |  |  |  |  |  |
| Na+, Si, Mg2+ |  |  |  |  |  | |
| K+ |  |  |  |  |  | |
| SO42− |  |  |  |  |  | |
| Cl−, NO3− |  |  |  |  |  | |
| DOC |  |  |  |  |  | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulloa-Cedamanos, F.; Probst, A.; Dos-Santos, V.; Camboulive, T.; Granouillac, F.; Probst, J.-L. Stream Hydrochemical Response to Flood Events in a Multi-Lithological Karstic Catchment from the Pyrenees Mountains (SW France). Water 2021, 13, 1818. https://doi.org/10.3390/w13131818
Ulloa-Cedamanos F, Probst A, Dos-Santos V, Camboulive T, Granouillac F, Probst J-L. Stream Hydrochemical Response to Flood Events in a Multi-Lithological Karstic Catchment from the Pyrenees Mountains (SW France). Water. 2021; 13(13):1818. https://doi.org/10.3390/w13131818
Chicago/Turabian StyleUlloa-Cedamanos, Francesco, Anne Probst, Vanessa Dos-Santos, Thierry Camboulive, Franck Granouillac, and Jean-Luc Probst. 2021. "Stream Hydrochemical Response to Flood Events in a Multi-Lithological Karstic Catchment from the Pyrenees Mountains (SW France)" Water 13, no. 13: 1818. https://doi.org/10.3390/w13131818