Effects of Cattails and Hydraulic Loading on Heavy Metal Removal from Closed Mine Drainage by Pilot-Scale Constructed Wetlands
Abstract
:1. Introduction
2. Materials and Methods
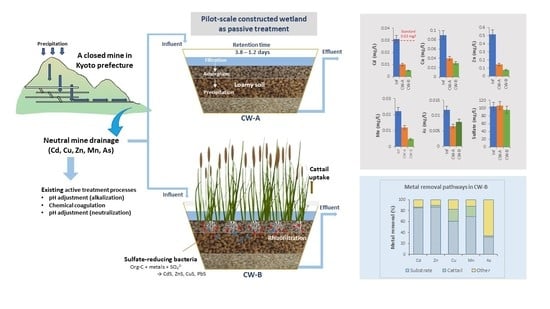
2.1. Pilot-Scale CW Setup and Operation
2.2. Sampling and Sample Preparation
2.3. Analytical Methods
2.4. Calculating Heavy Metal Removal and Accumulation
3. Results
3.1. Operational Conditions of the CWs
3.2. Water Parameters in the CWs
3.3. Heavy Metal Removal in CWs
3.4. Accumulation of Heavy Metals in Soil
3.5. Accumulation of Heavy Metals in Plant Biomass
3.6. Bacterial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez-Galán, M.; Moreno, F.M.B.; Vázquez, S.; Torralvo, F.A.; Vilches, L.F.; Zhang, Z. Remediation of acid mine drainage. Environ. Chem. Lett. 2019, 17, 1529–1538. [Google Scholar] [CrossRef]
- Ueda, H.; Masuda, N. An analysis on mine drainage treatment cost and the technical development to prevent mine pollution. Shigen-to-Sozai 2005, 121, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Acheampong, M.A.; Ansa, E.D.O. Low-cost technologies for mining wastewater treatment. J. Environ. Sci. Eng. B 2017, 6, 391–405. [Google Scholar] [CrossRef] [Green Version]
- Pat-Espadas, A.M.; Portales, R.L.; Amabilis-Sosa, L.E.; Gómez, G.; Vidal, G. Review of constructed wetlands for acid mine drainage treatment. Water 2018, 10, 1685. [Google Scholar] [CrossRef] [Green Version]
- Sheoran, A.; Sheoran, V. Heavy metal removal mechanism of acid mine drainage in wetlands: A critical review. Miner. Eng. 2006, 19, 105–116. [Google Scholar] [CrossRef]
- Sobolewski, P.A. A review of processes responsible for metal removal in wetlands treating contaminated mine drainage. Int. J. Phytoremediation 1999, 1, 19–51. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, Y.; Zhou, J.; Tang, Z.; Li, L.; Zhou, Q.; Vymazal, J. Effects of cattail biomass on sulfate removal and carbon sources competition in subsurface-flow constructed wetlands treating secondary effluent. Water Res. 2014, 59, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Park, S.S.; Jaffe, P.R. The effect of emergent macrophytes on the dynamics of sulfur species and trace metals in wetland sediments. Environ. Pollut. 2006, 140, 286–293. [Google Scholar] [CrossRef]
- Liu, J.-G.; Li, G.-H.; Shao, W.-C.; Xu, J.-K.; Wang, D.-K. Variations in uptake and translocation of copper, chromium and nickel among nineteen wetland plant species. Pedosphere 2010, 20, 96–103. [Google Scholar] [CrossRef]
- Eger, P. Wetland treatment for trace metal removal from mine drainage: The importance of aerobic and anaerobic processes. Water Sci. Technol. 1994, 29, 249–256. [Google Scholar] [CrossRef]
- Sasaki, K.; Hori, O.; Ogino, T.; Takano, K.; Endo, Y.; Tsunekawa, M.; Hirajima, T. Treatment of heavy metals in a constructed wetland, Kaminokuni, Hokkaido-Role of microorganisms in immobilization of heavy metals in wetland soils-. J. MMIJ 2009, 125, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Ogino, T.; Hori, O.; Endo, Y.; Kurosawa, K.; Tsunekawa, M. Chemical transportation of heavy metals in the constructed wetland impacted by acid drainage. Mater. Trans. 2003, 44, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, K.; Ogino, T.; Hori, O.; Takano, K.; Endo, Y.; Sakurai, Y.; Irie, K. Treatment of heavy metals in a constructed wetland, Kaminokuni, Hokkaido-Accumulation of heavy metals in emergent vegetations-. J. MMIJ 2009, 125, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Kato, T.; Kawasaki, Y.; Kadokura, M.; Suzuki, K.; Tawara, Y.; Ohara, Y.; Tokoro, C. Application of GETFLOWS coupled with chemical reactions to arsenic removal through ferrihydrite coprecipitation in an artificial wetland of a Japanese closed mine. Minerals 2020, 10, 475. [Google Scholar] [CrossRef]
- Soda, S.; Sasaki, R.; Nguyen, T.T.; Hayashi, K.; Kanayama, A. A laboratory experiment system for developing mine drainage treatment technologies using constructed wetlands—Sequencing batch treatment of Cd-containing neutral mine drainage—. Resour. Process. 2021, 67, 111–116. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewateri, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012; ISBN 978-0875532356. [Google Scholar]
- Postgate, J.R. The Sulphate Reducing Bacteria, 2nd ed.; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.; Al-Ghalith, G.A.; Caporaso, J.G. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. PeerJ 2018, 37, 852–857. [Google Scholar] [CrossRef]
- McIlroy, S.J.; Saunders, A.; Albertsen, M.; Nierychlo, M.; McIlroy, B.; Hansen, A.A.; Karst, S.M.; Nielsen, J.L.; Nielsen, P.H. MiDAS: The field guide to the microbes of activated sludge. Database 2015, 2015, bav062. [Google Scholar] [CrossRef]
- Dan, A.; Oka, M.; Fujii, Y.; Soda, S.; Ishigaki, T.; Machimura, T.; Ike, M. Removal of heavy metals from synthetic landfill leachate in lab-scale vertical flow constructed wetlands. Sci. Total. Environ. 2017, 584–585, 742–750. [Google Scholar] [CrossRef]
- Soda, S.; Hamada, T.; Yamaoka, Y.; Ike, M.; Nakazato, H.; Saeki, Y.; Kasamatsu, T.; Sakurai, Y. Constructed wetlands for advanced treatment of wastewater with a complex matrix from a metal-processing plant: Bioconcentration and translocation factors of various metals in Acorus gramineus and Cyperus alternifolius. Ecol. Eng. 2012, 39, 63–70. [Google Scholar] [CrossRef]
- Klink, A.; Macioł, A.; Wisłocka, M.; Krawczyk, J. Metal accumulation and distribution in the organs of Typha latifolia L. (cattail) and their potential use in bioindication. Limnologica 2013, 43, 164–168. [Google Scholar] [CrossRef]
- Hiller, K.A.; Foreman, K.H.; Weisman, D.; Bowen, J.L. Permeable reactive barriers designed to mitigate eutrophication alter bacterial community composition and aquifer redox conditions. Appl. Environ. Microbiol. 2015, 81, 7114–7124. [Google Scholar] [CrossRef] [Green Version]
- Castro, H.F.; Williams, N.H.; Ogram, A. Phylogeny of sulfate-reducing bacteria1. FEMS Microbiol. Ecol. 2000, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Hamai, T.; Hori, T.; Sato, Y.; Kobayashi, M.; Sato, Y.; Inaba, T.; Ogata, A.; Habe, H.; Sakata, T. Microbial community analysis of sulfate-reducing passive bioreactor for treating acid mine drainage under failure conditions after long-term continuous operation. J. Environ. Chem. Eng. 2018, 6, 5795–5800. [Google Scholar] [CrossRef]
- Kuever, J. The Family Desulfohalobiaceae, The Prokaryotes; Springer: Berlin/Heidelberg, Germany, 2014; pp. 87–95. [Google Scholar]
- Peng, W.; Li, X.; Liu, T.; Liu, Y.; Ren, J.; Liang, D.; Fan, W. Biostabilization of cadmium contaminated sediments using indigenous sulfate reducing bacteria: Efficiency and process. Chemosphere 2018, 201, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.D.; Leite, L.R.; Cuadros-Orellana, S.; Oliveira, G. Taxonomic and functional diversity of microbial community from a mining environment. BMC Bioinform. 2015, 16, A3. [Google Scholar] [CrossRef] [Green Version]
- Bhakta, J.N.; Munekage, Y. Identification of potential soil adsorbent for the removal of hazardous metals from aqueous phase. Int. J. Environ. Sci. Technol. 2012, 10, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Reeves, R.D. Tropical hyperaccumulators of metals and their potential for phytoextraction. Plant Soil 2003, 249, 57–65. [Google Scholar] [CrossRef]







| Element | Wetland | Phase I | Phase II | Phase III | Phase IV | Total |
|---|---|---|---|---|---|---|
| Cd | CW-A | 81.1 ± 8.2 | 77.3 ± 5.5 | 72.4 ± 10.9 | 69.4 ± 6.3 | 74.3 ± 8.9 |
| CW-B | 91.2 ± 5.2 | 87.5 ± 3.6 | 81.8 ± 5.9 | 82.9 ± 3.7 | 84.5 ± 5.8 | |
| Zn | CW-A | 83.1 ± 8.8 | 80.1 ± 8.9 | 74.0 ± 9.7 | 73.1 ± 6.3 | 76.9 ± 8.9 |
| CW-B | 92.1 ± 4.3 | 87.8 ± 9.1 | 81.3 ± 12.1 | 85.9 ± 4.0 | 86.6 ± 8.2 | |
| Cu | CW-A | 80.0 ± 18.1 | 74.9 ± 22.4 | 54.9 ± 18.1 | 45.6 ± 8.7 | 61.2 ± 21.3 |
| CW-B | 90.2 ± 6.9 | 75.9 ± 22.7 | 50.5 ± 11.9 | 50.2 ± 8.3 | 64.3 ± 21.2 | |
| Fe | CW-A | 77.2 ± 23.6 | 52.4 ± 43.0 | 22.7 ± 56.8 | −10.0 ± 36.6 | 22.1 ± 51.6 |
| CW-B | 97.3 ± 3.1 | 52.0 ± 36.5 | 7.4 ± 48.5 | −64.0 ± 99.1 | −1.4 ± 89.2 | |
| Mn | CW-A | −24.7 ± 54.2 | 47.3 ± 45.2 | 93.4 ± 11.2 | 86.3 ± 12.9 | 54.5 ± 57.6 |
| CW-B | 37.4 ± 57.3 | 87.8 ± 9.9 | 98.6 ± 3.7 | 99.3 ± 1.7 | 82.2 ± 37.4 | |
| As | CW-A | 83.0 ± 6.8 | 75.4 ± 37.3 | 51.7 ± 85.7 | 32.6 ± 64.4 | 60.6 ± 57.9 |
| CW-B | 60.8 ± 17.0 | 95.9 ± 5.7 | 9.6 ± 81.7 | 18.5 ± 62.6 | 43.3 ± 60.9 |
| Element | BCF | TF | |
|---|---|---|---|
| CW-B | Before Use | ||
| Cd | 2.29 | 0.80 | 0.86 |
| Zn | 3.03 | 0.49 | 0.44 |
| Cu | 4.69 | 0.49 | 0.41 |
| Fe | 0.85 | 0.41 | 0.11 |
| Mn | 0.67 | 0.67 | 0.66 |
| As | 0.87 | 0.81 | 0.51 |
| Family/Genus species | OTU_No | Influent (NMD) | Effluent CW-A | Effluent CW-B | Soil CW-A | Soil CW-B |
|---|---|---|---|---|---|---|
| Desulfovibrionaceae | OUT754 | 0 | 0 | 0 | 0 | 0.19 |
| Desulfovibrio mexicanus | OUT683, OTU1685 | 0 | 0 | 0 | 0 | 0.29 |
| Syntrophaceae Desulfomonile | OTU1970 | 0 | 0 | 0 | 0 | 0.05 |
| Desulfarculaceae | OTU2672 | 0 | 0 | 0 | 0 | 0.03 |
| Desulfobacteraceae | OTU1430 | 0 | 0 | 0 | 0 | 0.09 |
| Desulfobulbaceae Desulfobulbus | OTU1330 | 0 | 0 | 0 | 0 | 0.1 |
| Desulfobulbaceae | OTU3199, OTU2681, OTU1812, OTU1826 | 0 | 0 | 0 | 0 | 0.17 |
| Thermodesulfovibrionaceae | OTU3078, OTU2773, OTU401, OTU2368, OTU2901, OTU3191 | 0.24 | 0.14 | 0.05 | 0 | 0.01 |
| Total (%) | 0.24 | 0.14 | 0.05 | 0 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Soda, S.; Kanayama, A.; Hamai, T. Effects of Cattails and Hydraulic Loading on Heavy Metal Removal from Closed Mine Drainage by Pilot-Scale Constructed Wetlands. Water 2021, 13, 1937. https://doi.org/10.3390/w13141937
Nguyen TT, Soda S, Kanayama A, Hamai T. Effects of Cattails and Hydraulic Loading on Heavy Metal Removal from Closed Mine Drainage by Pilot-Scale Constructed Wetlands. Water. 2021; 13(14):1937. https://doi.org/10.3390/w13141937
Chicago/Turabian StyleNguyen, Thuong Thi, Satoshi Soda, Akihiro Kanayama, and Takaya Hamai. 2021. "Effects of Cattails and Hydraulic Loading on Heavy Metal Removal from Closed Mine Drainage by Pilot-Scale Constructed Wetlands" Water 13, no. 14: 1937. https://doi.org/10.3390/w13141937