Microplastics Investigation Using Zooplankton Samples from the Coasts of Cyprus (Eastern Mediterranean)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Microplastic Fibers
3.2. Microplastic Fragments
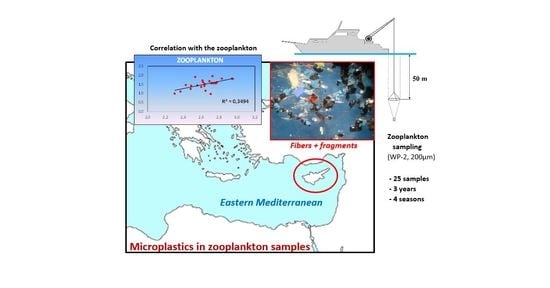
3.3. Correlation with the Zooplankton
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choy, C.A.; Robison, B.H.; Gagne, T.O.; Erwin, B.; Firl, E.; Halden, R.U.; Hamilton, J.A.; Katija, K.; Lisin, S.E.; Rolsky, C.; et al. The vertical distribution and biological transport of marine microplastics across the epipelagic and mesopelagic water column. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Gorokhova, E. Screening for microplastic particles in plankton samples: How to integrate marine litter assessment into existing monitoring programs? Mar. Pollut. Bull. 2015, 99, 271–275. [Google Scholar] [CrossRef]
- Collignon, A.; Hecq, J.H.; Galgani, F.; Collard, F.; Goffart, A. Annual variation in neustonic micro- and meso-plastic particles and zooplankton in the Bay of Calvi (Mediterranean-Corsica). Mar. Pollut. Bull. 2014, 79, 293–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Courtene-Jones, W.; Wang, X.; Song, Z.; Wei, N.; Li, D. Elucidating the vertical transport of microplastics in the water column: A review of sampling methodologies and distributions. Water Res. 2020, 186, 116403. [Google Scholar] [CrossRef] [PubMed]
- Pedrotti, M.L.; Mazzocchi, M.G.; Lombard, F.; Galgani, F.; Kerros, M.E.; Henry, M.; Elineau, A.; Petit, S.; Fernandez-de-Puelles, M.L.; Gasparini, S.; et al. TARA Mediterranean Expedition: Assessing the impact of microplastics on Mediterranean ecosystem. In Proceedings of the International Conference on Microplastic Pollution in the Mediterranean Sea, Capri, Italy, 26–29 September 2017; Cocca, M., Di Pace, E., Errico, M., Gentile, G., Montarsolo, A., Mossotti, R., Eds.; Springer Water: Cham, Switzerland. [Google Scholar] [CrossRef]
- Reisser, J.; Shaw, J.; Wilcox, C.; Hardesty, B.D.; Proietti, M.; Thums, M.; Pattiaratchi, C. Marine plastic pollution in waters around Australia: Characteristics, concentrations, and pathways. PLoS ONE 2013, 8, e80466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, P.G. A simple technique for counting marine debris at sea reveals steep litter gradients between the Straits of Malacca and the Bay of Bengal. Mar. Pollut. Bull. 2013, 69, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Orejon, L.F.; Sarda, R.; Ramis-Pujol, J. Floating plastic debris in the Central and Western Mediterranean Sea. Mar. Environ. Res. 2016, 120, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic polymers in Mediterranean surface waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef] [Green Version]
- Cozar, A.; Sanz-Martin, M.; Marti, E.; Gonzalez-Gordillo, J.I.; Ubeda, B.; Galvez, J.A.; Irigoien, X.; Duarte, C.M. Plastic accumulation in the Mediterranean Sea. PLoS ONE 2015, 10, e0121762. [Google Scholar] [CrossRef] [Green Version]
- Güven, O.; Gökdağ, K.; Jovanović, B.; Kıdeyş, A.E. Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environ. Pollut. 2017, 223, 286–294. [Google Scholar] [CrossRef]
- Gündoğdu, S.; Çevik, C. Micro-and mesoplastics in Northeast Levantine coast of Turkey: The preliminary results from surface samples. Mar. Pollut. Bull. 2017, 118, 341–347. [Google Scholar] [CrossRef]
- Van der Hal, N.; Ariel, A.; Angel, D.L. Exceptionally high abundances of microplastics in the oligotrophic Israeli Mediterranean coastal waters. Mar. Pollut. Bull. 2017, 116, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Kornilios, S.; Drakopoulos, P.G.; Dounas, C. Pelagic tar, dissolved/dispersed petroleum hydrocarbons and plastic distribution in the Cretan sea, Greece. Mar. Pollut. Bull. 1998, 36, 989–993. [Google Scholar] [CrossRef]
- Siokou, I.; Zervoudaki, S.; Velaoras, D.; Theocharis, A.; Christou, E.D.; Protopapa, M.; Pantazi, M. Mesozooplankton vertical patterns along an east-west transect in the oligotrophic Mediterranean Sea during early summer. Deep-Sea Res. Part II 2019, 164, 170–189. [Google Scholar] [CrossRef]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef]
- Desforges, J.P.W.; Galbraith, M.; Ross, P.S. Ingestion of microplastics by zooplankton in the Northeast Pacific Ocean. Arch. Environ. Contam. Toxicol. 2015, 69, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, G.M.; Vianna, T.M.P. Suspended microplastics in a highly polluted bay: Abundance, size, and availability for mesozooplankton. Mar. Pollut. Bull. 2018, 135, 256–265. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Otero, V.; Sobral, P. Evidence of microplastics in samples of zooplankton from Portuguese coastal waters. Mar. Environ. Res. 2014, 95, 89–95. [Google Scholar] [CrossRef]
- Doyle, M.J.; Watson, W.; Bowlin, N.M.; Sheavly, S.B. Plastic particles in coastal pelagic ecosystems of the Northeast Pacific ocean. Mar. Environ. Res. 2011, 71, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef] [PubMed]
- Bagaev, A.; Mizyuk, A.; Khatmullina, L.; Isachenko, I.; Chubarenko, I. Anthropogenic fibres in the Baltic Sea water column: Field data, laboratory and numerical testing of their motion. Sci. Total Environ. 2017, 599–600, 560–571. [Google Scholar] [CrossRef] [PubMed]
- HELCOM. Manual for Marine Monitoring in the COMBINE Programme of HELCOM. 2017. Available online: http://www.helcom.fi/Documents/Action%20areas/Monitoring%20and%20assessment/Manuals%20and%20Guidelines/Manual%20for%20Marine%20Monitoring%20in%20the%20COMBINE%20Programme%20of%20HELCOM.pdf (accessed on 15 May 2021).
- Baini, M.; Fossi, M.C.; Galli, M.; Caliani, I.; Campani, T.; Finoia, M.G.; Panti, C. Abundance and characterization of microplastics in the coastal waters of Tuscany (Italy): The application of the MSFD monitoring protocol in the Mediterranean Sea. Mar. Pollut. Bull. 2018, 133, 543–552. [Google Scholar] [CrossRef]
- Fossi, M.C.; Panti, C.; Guerranti, C.; Coppola, D.; Giannetti, M.; Marsili, L.; Panti, C.; de Sabata, E.; Clò, S. Are baleen whales exposed to the threat of microplastics? A case study of the Mediterranean fin whale (Balaenoptera physalus). Mar. Pollut. Bull. 2012, 64, 2374–2379. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.; Saraux, C.; Heitz, O.; Nowaczyk, A.; Bonnet, D. Microplastics FTIR characterisation and distribution in the water column and digestive tracts of small pelagic fish in the Gulf of Lions. Mar. Pollut. Bull. 2019, 142, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Sebille, E.; Wilcox, C.; Lebreton, L.; Maximenko, N.; Hardesty, B.D.; Van Franeker, J.A.; Eriksen, M.; Siegel, D.; Galgani, F.; Law, K.L. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015, 10, 124006. [Google Scholar] [CrossRef]
- Cole, M.; Webb, H.; Lindeque, P.K.; Fileman, E.S.; Halsband, C.; Galloway, T.S. Isolation of microplastics in biota-rich seawater samples and marine organisms. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reineccius, J.; Appelt, J.-S.; Hinrichs, T.; Kaiser, D.; Stern, J.; Prien, R.D.; Waniek, J.I. Abundance and characteristics of microfibers detected in sediment trap material from the deep subtropical North Atlantic Ocean. Sci. Total Environ. 2020, 738, 140354. [Google Scholar] [CrossRef] [PubMed]
- Mathalon, A.; Hill, P. Microplastic fibers in the intertidal ecosystem surrounding Halifax Harbor, Nova Scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef]
- Remy, F.; Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Lepoint, G. When microplastic is not plastic: The ingestion of artificial cellulose fibers by macrofauna living in seagrass Macrophytodetritus. Environ. Sci. Technol. 2015, 49, 11158–11166. [Google Scholar] [CrossRef] [PubMed]
- Cesa, F.S.; Turra, A.; Baruque-Ramos, J. Synthetic fibers as microplastics in the marine environment: A review from textile perspective with a focus on domestic washings. Sci. Total Environ. 2017, 598, 1116–1129. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, C.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Lusher, A.; Burke, A.; O’Connor, A.; Officer, R. Microplastic pollution in the Northeast Atlantic Ocean: Validated and opportunistic sampling. Mar. Pollut. Bull. 2014, 88, 325–333. [Google Scholar] [CrossRef]
- Castro, R.O.; Silva, M.L.; Marques, M.R.C.; de Araújo, F.V. Evaluation of microplastics in Jurujuba Cove, Niterói, RJ, Brazil, an area of mussels farming. Mar. Pollut. Bull. 2016, 110, 555–558. [Google Scholar] [CrossRef]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Pedrotti, M.L.; Petit, S.; Elineau, A.; Bruzaud, S.; Crebassa, J.-C.; Dumontet, B.; Marti, E.; Gorsky, G.; Cózar, A. Changes in the floating plastic pollution of the Mediterranean Sea in relation to the distance to land. PLoS ONE 2016, 11, e0161581. [Google Scholar] [CrossRef] [Green Version]
- Ryan, P.G.; Cole, G.; Spiby, K.; Nel, R.; Osborne, A.; Perold, V. Impacts of plastic ingestion on post-hatchling loggerhead turtles off South Africa. Mar. Pollut. Bull. 2016, 107, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Mansui, J.; Darmon, G.; Ballerini, T.; Canneyt, O.; Ourmieres, Y.; Miaud, C. Predicting marine litter accumulation patterns in the Mediterranean basin: Spatio-temporal variability and comparison with empirical data. Prog. Oceanogr. 2020, 182, 102268. [Google Scholar] [CrossRef]
- Lattin, G.L.; Moore, C.J.; Zellers, A.F.; Moore, S.L.; Weisberg, S.B. A comparison of neustonic plastic and zooplankton at different depths near the southern California shore. Mar. Pollut. Bull. 2004, 49, 291–294. [Google Scholar] [CrossRef]
- Moore, C.J.; Moore, S.L.; Leecaster, M.K.; Weisberg, S.B. A comparison of plastic and plankton in the North Pacific central gyre. Mar. Pollut. Bull. 2001, 42, 1297–1300. [Google Scholar] [CrossRef]
- Hannides, C.; Siokou, I.; Zervoudaki, S.; Frangoulis, C.; Lange, M. Mesozooplankton biomass and abundance in Cyprus coastal waters and comparison with the Aegean Sea (eastern Mediterranean). Mediterr. Mar. Sci. 2015, 16, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Zodiatis, G.; Lardner, R.; Hayes, D.R.; Georgiou, G.; Sofianos, S.; Skliris, N.; Lascaratos, A. Operational ocean forecasting in the Eastern Mediterranean: Implementation and evaluation. Ocean Sci. 2008, 4, 31–47. [Google Scholar] [CrossRef] [Green Version]









| Sample | HR | HF | SR | SF | ML | CL | Total |
|---|---|---|---|---|---|---|---|
| LA 8–17 | 0.21 | 0.31 | 0.14 | 0.00 | 0.03 | 0.03 | 0.72 |
| LA 3–18 | 0.54 | 0.13 | 0.07 | 0.00 | 1.08 | 0.13 | 1.96 |
| LA 7–18 | 0.46 | 0.98 | 0.05 | 0.10 | 0.00 | 0.15 | 1.75 |
| LA 11–18 | 0.35 | 0.39 | 0.12 | 0.08 | 0.04 | 0.24 | 1.22 |
| LA 3–19 | 1.03 | 0.41 | 0.31 | 0.00 | 0.00 | 0.00 | 1.75 |
| LA 7–19 | 0.15 | 0.53 | 0.08 | 0.00 | 0.08 | 0.00 | 0.83 |
| AM 7–17 | 0.45 | 0.45 | 0.25 | 0.00 | 0.00 | 0.15 | 1.30 |
| AM 12–17 | 1.37 | 3.59 | 0.00 | 0.00 | 0.00 | 0.42 | 5.39 |
| AM 3–18 | 0.10 | 0.76 | 0.10 | 0.00 | 0.00 | 0.00 | 0.96 |
| AM 8–18 | 0.52 | 1.38 | 0.17 | 0.00 | 0.00 | 0.09 | 2.16 |
| AM 11–18 | 1.08 | 0.83 | 0.17 | 0.17 | 0.08 | 0.58 | 2.92 |
| AM 4–19 | 0.26 | 0.66 | 0.00 | 0.04 | 0.30 | 0.04 | 1.29 |
| ME 7–17 | 0.88 | 0.96 | 0.16 | 0.00 | 0.00 | 0.16 | 2.15 |
| ME 11–17 | 0.71 | 1.29 | 0.35 | 0.12 | 0.24 | 0.00 | 2.71 |
| ME 3–18 | 0.98 | 0.98 | 0.42 | 0.00 | 0.00 | 0.00 | 2.39 |
| ME 7–18 | 0.50 | 0.32 | 0.13 | 0.13 | 0.00 | 0.00 | 1.07 |
| ME 2–19 | 17.17 | 2.28 | 0.22 | 0.54 | 0.00 | 0.11 | 20.33 |
| ME 5–19 | 0.67 | 1.35 | 0.38 | 0.00 | 0.38 | 0.00 | 2.79 |
| ME 9–19 | 0.77 | 0.77 | 0.14 | 0.07 | 0.00 | 0.21 | 1.96 |
| PR 7–17 | 0.74 | 3.09 | 0.62 | 0.12 | 0.00 | 0.37 | 4.94 |
| PR 10–17 | 0.30 | 1.80 | 0.10 | 0.00 | 0.20 | 0.00 | 2.40 |
| PR 4–18 | 0.10 | 0.58 | 0.19 | 0.68 | 0.29 | 0.19 | 2.04 |
| PR 8–18 | 0.35 | 0.18 | 0.00 | 0.00 | 0.00 | 0.44 | 0.96 |
| PR 2–19 | 30.67 | 0.56 | 0.00 | 0.00 | 2.58 | 0.11 | 33.93 |
| PR 6–19 | 0.42 | 1.56 | 0.52 | 1.25 | 0.94 | 0.10 | 4.79 |
| Total | 60.79 | 26.16 | 4.68 | 3.30 | 6.24 | 3.54 | 104.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilopoulou, G.; Kehayias, G.; Kletou, D.; Kleitou, P.; Triantafyllidis, V.; Zotos, A.; Antoniadis, K.; Rousou, M.; Papadopoulos, V.; Polykarpou, P.; et al. Microplastics Investigation Using Zooplankton Samples from the Coasts of Cyprus (Eastern Mediterranean). Water 2021, 13, 2272. https://doi.org/10.3390/w13162272
Vasilopoulou G, Kehayias G, Kletou D, Kleitou P, Triantafyllidis V, Zotos A, Antoniadis K, Rousou M, Papadopoulos V, Polykarpou P, et al. Microplastics Investigation Using Zooplankton Samples from the Coasts of Cyprus (Eastern Mediterranean). Water. 2021; 13(16):2272. https://doi.org/10.3390/w13162272
Chicago/Turabian StyleVasilopoulou, Grigoria, George Kehayias, Demetris Kletou, Periklis Kleitou, Vassilios Triantafyllidis, Anastasios Zotos, Konstantinos Antoniadis, Maria Rousou, Vassilis Papadopoulos, Polina Polykarpou, and et al. 2021. "Microplastics Investigation Using Zooplankton Samples from the Coasts of Cyprus (Eastern Mediterranean)" Water 13, no. 16: 2272. https://doi.org/10.3390/w13162272