1. Introduction
Water resources such as natural lakes and man-made reservoirs have been vital in supporting the increase in population growth, agricultural productivity, industrial activity, and economic development [
1]. Presently, a high pressure on water resources is present around the world due to the previous factors in unison with climate change and freshwater cultural eutrophication [
2]. This cultural eutrophication is mainly an input increment of nitrogen and phosphorus into waterbodies or catchment basin areas, and primarily caused by industrial activity [
3]. These high inputs and rising temperatures tend to intensify eutrophication and lead to cyanobacterial blooms, floating plant predominance, dissolved oxygen decrement, and, therefore, low water quality [
4].
The implementation of the European Water Framework Directive was one response to this situation. This directive presents the requirements and assessments to control the water quality and classify waterbodies into different “Ecological Status” throughout the European Union [
5]. The main aim of the Water Framework Directive (WFD) is to achieve a “good ecological status” in all waterbodies. This ecological status is obtained through hydro-morphological and physical–chemical indicators, as well as certain Biological Quality Elements (BQE). Included in the last indicators were phytoplankton, phytobenthos, macrophytes, benthic macroinvertebrates, and fish. However, and, surprisingly, without a scientifically based explanation, zooplankton was not included as a BQE [
6,
7,
8].
Zooplankton organisms have a fundamental position in aquatic food webs [
9] and are an important element in the structure and function of aquatic ecosystems. This is because they occupy the role of primary consumers and are the energy link between primary producers such as phytoplankton and higher consumers such as fish [
10]. Additionally, zooplankton can respond quickly to changes from trophic cascades, such as phytoplankton blooms, in a bottom-up process or top-down control, controlling and determining algae composition and abundance [
11,
12,
13]. Moreover, the zooplankton community responds to physical–chemical habitat conditions easily, which affects their species’ richness, increasing or decreasing densities, and promoting shifts in their diversity [
14,
15]. It is because of these characteristics that they can be a suitable indicator of water quality [
16,
17].
Worldwide, in waterbodies with different environmental conditions, several studies have used the whole zooplankton community as an indicator [
7,
18,
19,
20,
21] or used only specific zooplankton groups, such as rotifers or microcrustaceans [
22,
23,
24,
25]. Recently, within the Iberian Peninsula, the use of zooplankton species as indicators of trophic status in reservoirs has been evaluated in different basins such as Ebro [
26,
27], Cavado [
28], and Jucar [
29].
Studies focused on the use of zooplankton biomass, abundance, and ratios to determine trophic state have recently increased in several parts of the European Union [
8,
9,
30,
31,
32,
33]. However, in natural lakes [
34,
35,
36] and man-made reservoirs in the Mediterranean region, there are fewer studies [
37,
38]. Finally, studies on the use of zooplankton biomass and abundance as indicators of ecological status under the WDF criteria are scarce in scientific research.
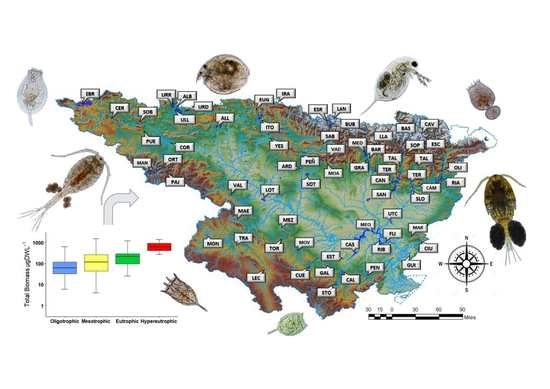
The aim of this study was to establish the value of the zooplankton metrics used in determining the trophic status and ecological potential for lentic waters. In the study, we used a robust data set collected over the last ten years in 66 reservoirs along the Ebro watershed, involving more than 300 sampling events over 10 years. The present research contributes to the research on zooplankton as a useful indicator to determine the trophic state and ecological potential within the context of the WFD requirements.
4. Discussion
The current study presents data from reservoirs widely distributed at the Ebro watershed, located in the Mediterranean area. It presents high variation of environmental data (most of the different trophic states and ecological potentials were present) indicating the high heterogeneity across the sampled reservoirs. Through the statistical treatment applied in the present research to the large dataset, we were able to define the zooplankton metrics that are related to environmental conditions and could be used to determine trophic status and ecological potential.
Several environmental variables are of a high importance in determining the water quality in lentic waters. One of these is the concentration of TP (a classical bottom-up variable). TP was correlated with a high number of the zooplankton metrics that were proposed in the current study. However, the chlorophyll a was the variable most correlated with metrics, in constrast with dissolved oxygen, which was not related with many metrics. Nevertheless, not only did environmental data vary, zooplankton metrics also showed different patterns through reservoirs and trophic gradients.
The total abundance of zooplankton as indicator of trophic state has been tested in another Iberian watershed [
38], and as a water quality tool in natural Mediterranean lakes [
36], both exhibiting an increment in zooplankton density along with both eutrophication and ecological potential increase, similarly to our results. Although biomass is dependent on abundance, it is an interesting metric to consider, because the change in any group dominance can mean high changes in the biomass and an increment in the percentage of microcrustaceans in relation to rotifers and zooplankton biomass has been linked to TP in different climatic zones [
54]. Biomass increased along the trophic gradient but showed a strong pattern when compared to abundances, as observed in European lakes [
33,
36]. Both density as well as biomass were statistically significant in differentiating between almost all water quality classes; however, biomass was slightly better to separate oligotrophic from mesotrophic environments. Nevertheless, both metrics, abundance and biomass could be used to determine bad or good water quality.
Normally, most of the total biomass is comprised of large-bodied zooplankton (mainly microcrustaceans), and thus, large-bodied biomass is very similar to total biomass. This is unlike total abundance, which may differ in the presence of small-bodied zooplankton. Gyllström et al. [
54] reported a positive relationship between the increment of large zooplankton biomass and TP. In the current study, we found a similar tendency along the trophic gradient and ecological potential. Additionally, the density of the large zooplankton presented similar patterns and could distinguish marginally better between ecological potentials than biomasses.
Small-bodied zooplankton, mainly composed of rotifers and small cladocerans, were correlated with variables that determined the trophic status, particularly with Chl-
a. These small zooplankton can shape the community in terms of abundance, especially for the high number of rotifer individuals [
10,
22]. These metrics were effective in distinguishing between low and high productive waters in general, but less sensible than other metrics in differentiating precisely between consecutive levels. A high elevate abundance and biomass could indicate an increment in the trophic gradient or other environmental pressures [
54].
An important aspect related to the zooplankton community is the high pressure from planktivorous fish [
57], which strongly affect zooplankton abundance, biomass, community composition, and even the size of the structure, especially in low productive waters [
58,
59,
60]. On the other hand, phytoplankton blooms, especially cyanobacterial blooms, can become an additional pressure [
61]. Cyanobacteria are a poor-quality food resource [
62], produce harmful cyanotoxins [
63,
64], and can promote zooplankton community shift into small-bodied species [
65,
66,
67]. In comparison to large-bodied species, small species are less mechanically affected (clogging of their filtering apparatus) by the presence of cyanobacterial colonies, mainly of filamentous forms [
68] in habitats with a eutrophic increment, where such blooms normally surge [
59]. Hence, the use of large and small zooplankton as a metric could provide valuable data about both fish and cyanobacterial pressures.
The crustacean index used in Polish lakes is the NCRU [
32]; however, it is inclusive of all crustaceans in general. In our study, in order to detect possible variations, we decided to divide them into groups. In our results, calanoid copepod abundances and biomass increased marginally from oligotrophic to eutrophic systems, but normally, at high trophic status, calanoids decreased; for this reason, they are typically considered to be from oligotrophic waters [
25,
55]. Cyclopoids showed a considerable increment, especially inside eutrophic and hypereutrophic reservoirs; lately, their presence and the occurrence of
Acanthocyclops species are used as indicators of eutrophic conditions in Mediterranean waterbodies [
26,
27,
29,
34]. For this study in general, the use of copepod metrics, including both calanoids and cyclopoids, resulted in the same correlation between environmental variables, and was a reliable indicator to differentiate between low and high productive waters. However, these metrics combined with the use of an indicator species could lead to a better water quality classification.
The Cladocera and daphnids metrics were very similar in that they were both effective differentiating among the trophic status levels; however, in ecological potential, only the Cladocera metric had the ability to detect between good and moderate levels. This lack of Daphnia sensitivity was probably because most reservoirs were classified as both good or superior and moderate, and had a wide value range in environmental variables such as TP and Chl-a. Another reason for the differences between metrics could be the presence or absence of Diaphanosoma spp. and Holopedium spp. as the dominant Cladocera inside reservoirs.
In several European waterbodies, there was a positive relationship between TP concentration and
Daphnia spp. in different waterbodies; at higher TP concentration a biomass increase has been reported [
54,
59]. This is in accordance with our results: in lower trophic levels, daphnids abundance and biomass were lower, and there was an increase at higher levels. Furthermore, it is important to point out that within this group, there is a species that considered to be an indicator for both low (
Daphnia longispina) and high eutrophic levels (
Daphnia cucullata) [
26,
27,
37]. These species were present only in reservoirs with low and high trophic status, respectively.
In eutrophic reservoirs, an increase in rotifers was detected in comparison with those of oligotrophic status [
69]. These findings were in concurrence with our results; however, rotifer metrics had the lesser correlation with environmental variables, and they were not sensitive enough to indicate separation in trophic status or ecological levels compared to other metrics. Nevertheless, in natural lakes, this group is a useful tool to determine the trophic status using abundances [
30,
31] or biomass [
31,
33]. Moreover, not only biomass and abundances are related to trophic gradient, but several studies in natural and man-made waterbodies have pointed to the rotifer species as being good indicators of trophic status and water quality [
22,
26,
27,
29,
35,
37,
70]; therefore, this group can provide valuable data and should not be overlooked.
An indicator of the cascading effects that zooplankton have on phytoplankton is the ZOO:PHYTO ratio [
8]. Previous studies in Danish lakes [
55,
59] and shallow sub-tropical lakes [
71] indicate that this ratio decrease coincides with an increase in TP. Here, we recorded a slight downward trend in this ratio along TP increase, although, we did not find a significant correlation; this is in accordance with results obtained in the Jucar watershed [
38]. These previous studies used the ZOO:PHYTO biomass, but, in our results, the use of biomass was not capable of differentiating between levels in trophic and ecological potential, whereas, the use of abundances could detect between low and high productive reservoirs.
A similar metric is the ZOO:CHLA ratio, using the measurement of phytoplankton production. In our study, the ZOO:CHLA ratio was very sensitive to changes in trophic gradient and, along with ZOO and CLAD, was the most effective metric differentiating most levels of both trophic state and ecological potential. They were also closely related to variables that determine water quality. An advantage of the ZOO:CHLA ratio is that it is not imperative that one be an expert zooplankton taxonomist, since it is only necessary to identify and count the major groups in general without reaching the species level, and Chl-a data can be obtained through any method to apply this metric.
The decrease in both ZOO:CHLA and ZOO:PHYTO metrics along the increase in the trophic gradient could be explained by bottom-up effect [
72]; the abundance and biomass of phytoplankton can change due to several variables such as cyanobacterial blooms, typically during summertime in productive waters with high levels of TP, and the replacement of edible phytoplankton with inedible and low quality species. Fish exert an additional pressure on zooplankton, especially in warmer waters [
54].
Zooplankton metrics such as density, biomass, large body size, cladocerans, and the ZOO:CHLA ratio can be used as good overall indicators to differentiate between trophic state and ecological potential levels. Metrics related to copepods, as well as their division in their orders, calanoids and cyclopoids, are especially sensible for distinguishing reservoirs with better water quality (such as oligotrophic from mesotrophic), trophic state, and good or higher from moderate in ecological potential.
The aim of the WFD was to bring European waterbodies up to good ecological levels. According to current research, most zooplankton metrics have been shown to be good indicators to differentiate between reservoirs that have good ecological potential and others. The integrative capacity of zooplankton with the environmental factors that determine the trophic state and the ecological potential, can give us a broader picture over time compared to phytoplankton. Due to their shorter lifespan, as well as their community composition, phytoplankton can change in a short time compared to zooplankton [
73], and even under specific environmental pressures or blooms, the phytoplankton could not give data as accurate. Thus, the use of the different zooplankton metrics presented here, along with indicator species, can be used as a tool to determine the water’s quality. Zooplankton collection does not present a great impact on budget nor working time because it can be sampled in parallel with phytoplankton. Phytoplankton sampling is included in monitoring programs, hence, the use of both plankton components is reasonable, in addition to being complementary, and could give us more precise water quality information. Finally, as several authors have recently reported, zooplankton can be a good indicator to determine both trophic status and ecological potential. Therefore, we strongly recommend that zooplankton be incorporated as one more BQE within the Water Framework Directive.