Tail Coiling Assay in Zebrafish (Danio rerio) Embryos: Stage of Development, Promising Positive Control Candidates, and Selection of an Appropriate Organic Solvent for Screening of Developmental Neurotoxicity (DNT)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Test Solutions
2.2. Zebrafish Husbandry and Egg Acquisition
2.3. Test Design
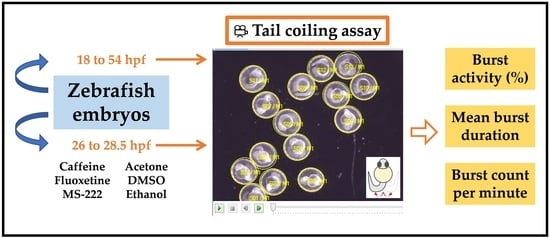
2.4. Tail Coiling Assay
2.5. Statistical Analysis
3. Results
3.1. Stage of Development
3.2. Positive Control Candidates
3.3. Organic Solvents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rice, D.; Barone, S., Jr. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar] [CrossRef]
- Barone, S., Jr.; Das, K.P.; Lassiter, T.L.; White, L.D. Vulnerable processes of nervous system development: A review of markers and methods. Neurotoxicology 2000, 21, 15–36. [Google Scholar] [PubMed]
- Bal-Price, A.; Pistollato, F.; Sachana, M.; Bopp, S.K.; Munn, S.; Worth, A. Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods. Toxicol. Appl. Pharmacol. 2018, 354, 7–18. [Google Scholar] [CrossRef]
- US Environmental Protection Agency (EPA). Health Effects Test Guidelines: Developmental Neurotoxicity Study; OPPTS 870.6300, EPA Document 712-C-98-239; EPA: Washington, DC, USA, 1998.
- OECD. Guidelines for the Testing of Chemicals, Section 4, Health Effects; Test No. 426: Developmental Neurotoxicity Study; OECD: Paris, France, 2007. [Google Scholar]
- Fritsche, E.; Grandjean, P.; Crofton, K.M.; Aschner, M.; Goldberg, A.; Heinonen, T.; Hessel, E.V.S.; Hogberg, H.T.; Bennekou, S.H.; Lein, P.J.; et al. Consensus statement on the need for innovation, transition and implementation of developmental neurotoxicity (DNT) testing for regulatory purposes. Toxicol. Appl. Pharmacol. 2018, 354, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Terron, A.; Bennekou, S.H. Towards a regulatory use of alternative developmental neurotoxicity testing (DNT). Toxicol. Appl. Pharmacol. 2018, 354, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, C. Developmental neurotoxicity test guidelines: Problems and perspectives. J. Toxicol. Sci. 2016, 41, SP69–SP79. [Google Scholar] [CrossRef] [Green Version]
- Fritsche, E.; Barenys, M.; Klose, J.; Masjosthusmann, S.; Nimtz, L.; Schmuck, M.; Wuttke, S.; Tigges, J. Current Availability of Stem Cell-Based In Vitro Methods for Developmental Neurotoxicity (DNT) Testing. Toxicol. Sci. 2018, 165, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christen, V.; Rusconi, M.; Crettaz, P.; Fent, K. Developmental neurotoxicity of different pesticides in PC-12 cells in vitro. Toxicol. Appl. Pharmacol. 2017, 325, 25–36. [Google Scholar] [CrossRef]
- Gassmann, K.; Abel, J.; Bothe, H.; Haarmann-Stemmann, T.; Merk, H.F.; Quasthoff, K.N.; Rockel, T.D.; Schreiber, T.; Fritsche, E. Species-specific differential AhR expression protects human neural progenitor cells against developmental neurotoxicity of PAHs. Environ. Health Perspect. 2010, 118, 1571–1577. [Google Scholar] [CrossRef] [Green Version]
- Bal-Price, A.K.; Hogberg, H.T.; Buzanska, L.; Lenas, P.; Van Vliet, E.; Hartung, T. In vitro developmental neurotoxicity (DNT) testing: Relevant models and endpoints. Neurotoxicology 2010, 31, 545–554. [Google Scholar] [CrossRef]
- Peterson, R.T.; Nass, R.; Boyd, W.A.; Freedman, J.H.; Dong, K.; Narahashi, T. Use of non-mammalian alternative models for neurotoxicological study. Neurotoxicology 2008, 29, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selderslaghs, I.W.; Hooyberghs, J.; De Coen, W.; Witters, H.E. Locomotor activity in zebrafish embryos: A new method to assess developmental neurotoxicity. Neurotoxicol. Teratol. 2010, 32, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Selderslaghs, I.W.; Hooyberghs, J.; Blust, R.; Witters, H.E. Assessment of the developmental neurotoxicity of compounds by measuring locomotor activity in zebrafish embryos and larvae. Neurotoxicol. Teratol. 2013, 37, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, H.; Xiong, J.; Mehler, W.T.; You, J. Developmental Toxicity of a Neonicotinoid Insecticide, Acetamiprid to Zebrafish Embryos. J. Agric. Food Chem. 2019, 67, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, L.; Liao, X.; Meng, Z.; Xiao, J.; Li, F.; Zhang, S.; Cao, Z.; Lu, H. Toxic effects of oxine-copper on development and behavior in the embryo-larval stages of zebrafish. Aquat. Toxicol. 2019, 210, 242–250. [Google Scholar] [CrossRef]
- Cao, F.; Souders, C.L.; Li, P.; Pang, S.; Qiu, L.; Martyniuk, C.J. Developmental toxicity of the triazole fungicide cyproconazole in embryo-larval stages of zebrafish (Danio rerio). Environ. Sci. Pollut. Res. Int. 2019, 26, 4913–4923. [Google Scholar] [CrossRef]
- Velki, M.; Di Paolo, C.; Nelles, J.; Seiler, T.B.; Hollert, H. Diuron and diazinon alter the behavior of zebrafish embryos and larvae in the absence of acute toxicity. Chemosphere 2017, 180, 65–76. [Google Scholar] [CrossRef]
- Mu, X.; Pang, S.; Sun, X.; Gao, J.; Chen, J.; Chen, X.; Li, X.; Wang, C. Evaluation of acute and developmental effects of difenoconazole via multiple stage zebrafish assays. Environ. Pollut. 2013, 175, 147–157. [Google Scholar] [CrossRef]
- Ramlan, N.F.; Sata, N.S.A.M.; Hassan, S.N.; Bakar, N.A.; Ahmad, S.; Zulkifli, S.Z.; Abdullah, C.A.C.; Ibrahim, W.N.W. Time dependent effect of chronic embryonic exposure to ethanol on zebrafish: Morphology, biochemical and anxiety alterations. Behav. Brain Res. 2017, 332, 40–49. [Google Scholar] [CrossRef]
- Cheng, R.; Jia, Y.; Dai, L.; Liu, C.; Wang, J.; Li, G.; Yu, L. Tris (1,3-dichloro-2-propyl) phosphate disrupts axonal growth, cholinergic system and motor behavior in early life zebrafish. Aquat. Toxicol. 2017, 192, 7–15. [Google Scholar] [CrossRef]
- Usenko, C.Y.; Robinson, E.M.; Usenko, S.; Brooks, B.W.; Bruce, E.D. PBDE developmental effects on embryonic zebrafish. Environ. Toxicol. Chem. 2011, 30, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Valim Brigante, T.A.; Abe, F.R.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.S.; De Oliveira, D.P. Cannabidiol did not induce teratogenicity or neurotoxicity in exposed zebrafish embryos. Chem. Biol. Interact. 2018, 291, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.; Salem, R.; Al-Asmakh, M.; Altamash, T.; Pintus, G.; Khraisheh, M.; Nasrallah, G.K. Toxicity evaluation of selected ionic liquid compounds on embryonic development of Zebrafish. Ecotoxicol. Environ. Saf. 2018, 161, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, C.; Zheng, L.; Simonich, M.; Bai, C.; Tanguay, R.; Dong, Q. Trimethyltin chloride (TMT) neurobehavioral toxicity in embryonic zebrafish. Neurotoxicol. Teratol. 2011, 33, 721–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.H.; Gao, J.M.; Huang, C.J.; Li, C.Q. Zebrafish models for assessing developmental and reproductive toxicity. Neurotoxicol. Teratol. 2014, 42, 35–42. [Google Scholar] [CrossRef]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Saint-Amant, L.; Drapeau, P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998, 37, 622–632. [Google Scholar] [CrossRef]
- Vliet, S.M.; Ho, T.C.; Volz, D.C. Behavioral screening of the LOPAC1280 library in zebrafish embryos. Toxicol. Appl. Pharmacol. 2017, 329, 241–248. [Google Scholar] [CrossRef]
- Zindler, F.; Beedgen, F.; Braunbeck, T. Time-course of coiling activity in zebrafish (Danio rerio) embryos exposed to ethanol as an endpoint for developmental neurotoxicity (DNT)—Hidden potential and underestimated challenges. Chemosphere 2019, 235, 12–20. [Google Scholar] [CrossRef]
- Raftery, T.D.; Isales, G.M.; Yozzo, K.L.; Volz, D.C. High-content screening assay for identification of chemicals impacting spontaneous activity in zebrafish embryos. Environ. Sci. Technol. 2014, 48, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Zindler, F.; Beedgen, F.; Brandt, D.; Steiner, M.; Stengel, D.; Baumann, L.; Braunbeck, T. Analysis of tail coiling activity of zebrafish (Danio rerio) embryos allows for the differentiation of neurotoxicants with different modes of action. Ecotoxicol. Environ. Saf. 2019, 186, 109754. [Google Scholar] [CrossRef] [PubMed]
- Ogungbemi, A.O.; Teixido, E.; Massei, R.; Scholz, S.; Küster, E. Optimization of the spontaneous tail coiling test for fast assessment of neurotoxic effects in the zebrafish embryo using an automated workflow in KNIME®. Neurotoxicol. Teratol. 2020, 81, 106918. [Google Scholar] [CrossRef] [PubMed]
- Crofton, K.M.; Foss, J.A.; Hass, U.; Jensen, K.F.; Levin, E.D.; Parker, S.P. Undertaking positive control studies as part of developmental neurotoxicity testing: A report from the ILSI Research Foundation/Risk Science Institute expert working group on neurodevelopmental endpoints. Neurotoxicol. Teratol. 2008, 30, 266–287. [Google Scholar] [CrossRef] [PubMed]
- Crofton, K.M.; Makris, S.L.; Sette, W.F.; Mendez, E.; Raffaele, K.C. A qualitative retrospective analysis of positive control data in developmental neurotoxicity studies. Neurotoxicol. Teratol. 2008, 26, 345–352. [Google Scholar] [CrossRef]
- Maes, J.; Verlooy, L.; Buenafe, O.E.; De Witte, P.A.; Esguerra, C.V.; Crawford, A.D. Evaluation of 14 organic solvents and carriers for screening applications in zebrafish embryos and larvae. PLoS ONE 2012, 7, e43850. [Google Scholar] [CrossRef]
- Sainio, M.A. Neurotoxicity of solvents. In Handbook of Clinical Neurology; Lotti, M., Bleecker, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 131, pp. 93–110. [Google Scholar] [CrossRef]
- ISO 4346-1. Water quality—Determination of the Acute Lethal Toxicity of Substances to a Freshwater Fish [Brachydanio Rerio Hamilton-Buchanan (Teleostei, Cyprinidae)]—Part 1: Static Method. 1996. Available online: https://www.iso.org/standard/14026.html (accessed on 6 January 2021).
- OECD Guideline for Testing Chemicals; Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013.
- Saint-Amant, L.; Drapeau, P. Synchronization of an embryonic network of identified spinal interneurons solely by electrical coupling. Neuron 2001, 31, 1035–1046. [Google Scholar] [CrossRef] [Green Version]
- Drapeau, P.; Saint-Amant, L.; Buss, R.R.; Chong, M.; McDearmid, J.R.; Brustein, E. Development of the locomotor network in zebrafish. Prog. Neurobiol. 2002, 68, 85–111. [Google Scholar] [CrossRef]
- Brustein, E.; Saint-Amant, L.; Buss, R.R.; Chong, M.; McDearmid, J.R.; Drapeau, P. Steps during the development of the zebrafish locomotor network. J. Physiol. Paris 2003, 97, 77–86. [Google Scholar] [CrossRef]
- Knogler, L.D.; Ryan, J.; Saint-Amant, L.; Drapeau, P. A hybrid electrical/chemical circuit in the spinal cord generates a transient embryonic motor behavior. J. Neurosci. 2014, 34, 9644–9655. [Google Scholar] [CrossRef] [Green Version]
- McKeown, K.A.; Downes, G.B.; Hutson, L.D. Modular laboratory exercises to analyze the development of zebrafish motor behavior. Zebrafish 2009, 6, 179–185. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, Y.; Ye, J.; Huang, C.; Zhao, M.; Liu, W. Dual enantioselective effect of the insecticide bifenthrin on locomotor behavior and development in embryonic-larval zebrafish. Environ. Toxicol. Chem. 2010, 29, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, C.; Wang, L.; Ye, X.; Bai, C.; Simonich, M.T.; Tanguay, R.L.; Dong, Q. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat. Toxicol. 2010, 98, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehlig, A.; Daval, J.L.; Debry, G. Caffeine and the central nervous system: Mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Rev. 1992, 17, 139–170. [Google Scholar] [CrossRef]
- Fisone, G.; Borgkvist, A.; Usiello, A. Caffeine as a psychomotor stimulant: Mechanism of action. Cell. Mol. Life Sci. 2004, 61, 857–872. [Google Scholar] [CrossRef]
- Rodriguez, R.S.; Haugen, R.; Rueber, A.; Huang, C.C. Reversible neuronal and muscular toxicity of caffeine in developing vertebrates. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 163, 47–54. [Google Scholar] [CrossRef]
- Perez-Caballero, L.; Torres-Sanchez, S.; Bravo, L.; Mico, J.A.; Berrocoso, E. Fluoxetine: A case history of its discovery and preclinical development. Expert Opin. Drug Discov. 2014, 9, 567–578. [Google Scholar] [CrossRef] [Green Version]
- Parolini, M.; Ghilardi, A.; De Felice, B.; Del Giacco, L. Environmental concentration of fluoxetine disturbs larvae behavior and increases the defense response at molecular level in zebrafish (Danio rerio). Environ. Sci. Pollut. Res. Int. 2019, 26, 34943–34952. [Google Scholar] [CrossRef]
- De Farias, N.O.; Oliveira, R.; Sousa-Moura, D.; De Oliveira, R.C.S.; Rodrigues, M.A.C.; Andrade, T.S.; Domingues, I.; Camargo, N.S.; Muehlmann, L.A.; Grisolia, C.K. Exposure to low concentration of fluoxetine affects development, behaviour and acetylcholinesterase activity of zebrafish embryos. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 215, 1–8. [Google Scholar] [CrossRef]
- Airhart, M.J.; Lee, D.H.; Wilson, T.D.; Miller, B.E.; Miller, M.N.; Skalko, R.G. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC). Neurotoxicol. Teratol. 2007, 29, 652–664. [Google Scholar] [CrossRef]
- Carter, K.M.; Woodley, C.M.; Brown, R.S. A review of tricaine methanesulfonate for anesthesia of fish. Rev. Fish Biol. Fish. 2011, 21, 51–59. [Google Scholar] [CrossRef]
- Menelaou, E.; Husbands, E.E.; Pollet, R.G.; Coutts, C.A.; Ali, D.W.; Svoboda, K.R. Embryonic motor activity and implications for regulating motoneuron axonal pathfinding in zebrafish. Eur. J. Neurosci. 2008, 28, 1080–1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hallare, A.; Nagel, K.; Köhler, H.R.; Triebskorn, R. Comparative embryotoxicity and proteotoxicity of three carrier solvents to zebrafish (Danio rerio) embryos. Ecotoxicol. Environ. Saf. 2006, 63, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Chromcova, L.; Stepanova, S.; Plhalova, L.; Praskova, E.; Svobodova, Z. Effect of four selected carrier solvents on embryonal stages of Danio rerio. Neuro Endocrinol. Lett. 2012, 33, 60–65. [Google Scholar]
- Likhodii, S.; Nylen, K.; Burnham, W.M. Acetone as an anticonvulsant. Epilepsia 2008, 49, 83–86. [Google Scholar] [CrossRef]
- Martinez, C.S.; Feas, D.A.; Siri, M.; Igartúa, D.E.; Chiaramoni, N.S.; Alonso, S.D.V.; Prieto, M.J. In vivo study of teratogenic and anticonvulsant effects of antiepileptics drugs in zebrafish embryo and larvae. Neurotoxicol. Teratol. 2018, 66, 17–24. [Google Scholar] [CrossRef]
- Kais, B.; Schneider, K.E.; Keiter, S.; Henn, K.; Ackermann, C.; Braunbeck, T. DMSO modifies the permeability of the zebrafish (Danio rerio) chorion-implications for the fish embryo test (FET). Aquat. Toxicol. 2013, 140–141, 229–238. [Google Scholar] [CrossRef]






| Positive Control Candidates | ||
| CAS 1 number | Concentration | |
| Caffeine | 58-08-2 | 150, 300, and 600 mg/L |
| Fluoxetine | 56296-78-7 | 10, 100, and 500 µg/L |
| MS-222 2 | 886-86-2 | 10, 50, and 100 mg/L |
| Organic solvents | ||
| CAS number | Concentration | |
| Acetone | 67-64-1 | 0.1%, 0.5%, and 1.0% v/v |
| DMSO 3 | 67-68-5 | 0.01%, 0.01%, and 1.00% v/v |
| Ethanol | 64-17-5 | 0.25%, 0.50%, and 1.00% v/v |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, A.A.S.; Brigante, T.A.V.; Oliveira, D.P. Tail Coiling Assay in Zebrafish (Danio rerio) Embryos: Stage of Development, Promising Positive Control Candidates, and Selection of an Appropriate Organic Solvent for Screening of Developmental Neurotoxicity (DNT). Water 2021, 13, 119. https://doi.org/10.3390/w13020119
de Oliveira AAS, Brigante TAV, Oliveira DP. Tail Coiling Assay in Zebrafish (Danio rerio) Embryos: Stage of Development, Promising Positive Control Candidates, and Selection of an Appropriate Organic Solvent for Screening of Developmental Neurotoxicity (DNT). Water. 2021; 13(2):119. https://doi.org/10.3390/w13020119
Chicago/Turabian Stylede Oliveira, Andréia A. S., Tamires A. V. Brigante, and Danielle P. Oliveira. 2021. "Tail Coiling Assay in Zebrafish (Danio rerio) Embryos: Stage of Development, Promising Positive Control Candidates, and Selection of an Appropriate Organic Solvent for Screening of Developmental Neurotoxicity (DNT)" Water 13, no. 2: 119. https://doi.org/10.3390/w13020119