Bioelectricity Generation and Production of Ornamental Plants in Vertical Partially Saturated Constructed Wetlands
Abstract
:1. Introduction
2. Methodology
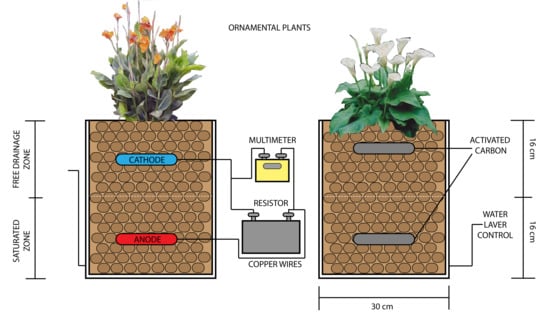
2.1. Description of Vertical Partially Saturated Constructed Wetlands
2.2. Production of Ornamental Plants in VPS-CWs
2.3. VPS-CW System Monitoring
2.4. Statistical Analysis
3. Results and Discussion
3.1. Production of Ornamental Plants in VPS-CWs
3.2. Contaminant Removal
3.3. Bioelectricity Production in VPS-CWs
Discussion: Bioelectricity Production in VPS-CWs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakase, C.; Zurita, F.; Nani, G.; Reyes, G.; Fernández-Lambert, G.; Cabrera-Hernández, A.; Sandoval, L. Nitrogen Removal from Domestic Wastewater and the Development of Tropical Ornamental Plants in Partially Saturated Mesocosm-Scale Constructed Wetlands. Int. J. Environ. Res. Public Health 2019, 16, 4800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, L.; Zhao, Y.; Zhao, X.; Hu, Y.; Hao, X.; Xu, L.; Liu, R. A review of a recently emerged technology: Constructed wetland–microbial fuel cells. Water Res. 2015, 85, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vargas, C.A.; Arias, C.A.; Zhang, L.; Paredes, D.; Brix, H. Community level physiological profiling of microbial electrochemical-based constructed wetlands. Sci. Total Environ. 2020, 137761. [Google Scholar] [CrossRef] [PubMed]
- Pelissari, C.; Ávila, C.; Trein, C.M.; García, J.; de Armas, R.D.; Sezerino, P.H. Nitrogen transforming bacteria within a full-scale partially saturated vertical subsurface flow constructed wetland treating urban wastewater. Sci. Total Environ. 2017, 574, 390–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelissari, C.; Guivernau, M.; Viñas, M.; García, J.; Velasco-Galilea, M.; Souza, S.S.; Ávila, C. Effects of partially saturated conditions on the metabolically active microbiome and on nitrogen removal in vertical subsurface flow constructed wetlands. Water Res. 2018, 141, 185–195. [Google Scholar] [CrossRef]
- Gikas, G.D.; Pérez-Villanueva, M.; Tsioras, M.; Alexoudis, C.; Pérez-Rojas, G.; Masís-Mora, M.; Tsihrintzis, V.A. Low-cost approaches for the removal of terbuthylazine from agricultural wastewater: Constructed wetlands and biopurification system. Chem. Eng. J. 2018, 335, 647–656. [Google Scholar] [CrossRef]
- Snyder, B.F. The Inclusion of Ecosystem Service Valuations in Bioenergy Cost Analysis: A Case Study of Constructed Wetlands in the Neotropics. Ecol. Econ. 2019, 156, 196–201. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Guo, F.; Liu, X.; Su, X.; He, Q. Metagenomic analysis of the biotoxicity of titanium dioxide nanoparticles to microbial nitrogen transformation in constructed wetlands. J. Hazard. Mater. 2020, 384, 121376. [Google Scholar] [CrossRef]
- Hu, B.; Hu, S.; Chen, Z.; Vymazal, J. Employ of arbuscular mycorrhizal fungi for pharmaceuticals ibuprofen and diclofenac removal in mesocosm-scale constructed wetlands. J. Hazard. Mater. 2020, 124524. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, F.; Luo, P.; Xiao, R.; Zhu, H.; Wu, J. Nitrous oxide emissions from pilot scale three-stage constructed wetlands with variable nitrogen loading. Bioresour. Technol. 2019, 289, 121687. [Google Scholar] [CrossRef]
- Saeed, T.; Majed, N.; Khan, T.; Mallika, H. Two-stage constructed wetland systems for polluted surface water treatment. J. Environ. Manag. 2019, 249, 109379. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U. Microbial fuel cells and microbial electrochemistry: Into the next century! ChemSusChem 2012, 5, 959. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, X.; Wang, Y.; Abayneh, B.; Ding, Y.; Yan, D.; Bai, J. Microbial community structure of different electrode materials in constructed wetland incorporating microbial fuel cell. Bioresour. Technol. 2016, 221, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Oon, Y.L.; Ong, S.A.; Ho, L.N.; Wong, Y.S.; Dahalan, F.A.; Oon, Y.S.; Nordin, N. Role of macrophyte and effect of supplementary aeration in up-flow constructed wetland-microbial fuel cell for simultaneous wastewater treatment and energy recovery. Bioresour. Technol. 2017, 224, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Cao, F.Q.; Kong, Q.; Zhou, L.L.; Yuan, Q.; Zhu, Y.J.; Wang, Q. Electricity production and evolution of microbial community in the constructed wetland-microbial fuel cell. Chem. Eng. J. 2018, 339, 479–486. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Ray, S.G.; Ghangrekar, M.M. Third generation in bio-electrochemical system research—A systematic review on mechanisms for recovery of valuable by-products from wastewater. Renew. Sustain. Energy Rev. 2017, 76, 1022–1031. [Google Scholar] [CrossRef]
- Corbella, C.; Puigagut, J. Improving domestic wastewater treatment efficiency with constructed wetland microbial fuel cells: Influence of anode material and external resistance. Sci. Total Environ. 2018, 631, 1406–1414. [Google Scholar] [CrossRef]
- Aguirre-Sierra, A.; Bacchetti-De Gregoris, T.; Salas, J.J.; de Deus, A.; Esteve-Núñez, A. A new concept in constructed wetlands: Assessment of aerobic electroconductive biofilters. Environ. Sci. Water Res. Technol. 2020, 6, 1312–1323. [Google Scholar] [CrossRef]
- Gude, V.G. Wastewater treatment in microbial fuel cells—An overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Lombard Latune, R.; Laporte-Daube, O.; Fina, N.; Peyrat, S.; Pelus, L.; Molle, P. Which plants are needed for a French vertical-flow constructed wetland under a tropical climate? Water Sci. Technol. 2017, 75, 1873–1881. [Google Scholar] [CrossRef]
- Walter, X.A.; Merino-Jiménez, I.; Greenman, J.; Ieropoulos, I. PEE POWER® urinal II—Urinal scale-up with microbial fuel cell scale-down for improved lighting. J. Power Sources 2018, 392, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, Y.; Fan, C.; Fan, Z.; Zhao, F. First study to explore the feasibility of applying microbial fuel cells into constructed wetlands for COD monitoring. Bioresour. Technol. 2017, 243, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Ni, B.J. Challenges in the application of microbial fuel cells to wastewater treatment and energy production: A mini review. Sci. Total Environ. 2018, 639, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Montiel, V.; Valero, D.; Gallud, F.; García-García, V.; Expósito, E.; Iniesta, J. Prospective Applications of Renewable Energy-Based Electrochemical Systems in Wastewater Treatment. In Electrochemical Water and Wastewater Treatment; Butterworth-Heinemann: Oxford, UK, 2018; pp. 513–541. [Google Scholar] [CrossRef]
- Hagen, M.; Dörfler, S.; Fanz, P.; Berger, T.; Speck, R.; Tübke, J.; Kaskel, S. Development and costs calculation of lithium–sulfur cells with high sulfur load and binder free electrodes. J. Power Sources 2013, 224, 260–268. [Google Scholar] [CrossRef]
- Lawry, S.; Samii, C.; Hall, R.; Leopold, A.; Hornby, D.; Mtero, F. The impact of land property rights interventions on investment and agricultural productivity in developing countries: A systematic review. J. Dev. Eff. 2017, 9, 61–81. [Google Scholar] [CrossRef] [Green Version]
- Mateo, N.; Nani, G.; Montiel, W.; Nakase, C.; Salazar-Salazar, C.; Sandoval, L. Effect of Canna Hibryds in Wetlands Built Specifically Saturated for the Treatment of Pig Waters. Rinderesu 2020, 4, 59–68. Available online: http://rinderesu.com/index.php/rinderesu/article/view/41 (accessed on 1 October 2020).
- Di, L.; Li, Y.; Nie, L.; Wang, S.; Kong, F. Influence of plant radial oxygen loss in constructed wetland combined with microbial fuel cell on nitrobenzene removal from aqueous solution. J. Hazard. Mater. 2020, 122542. [Google Scholar] [CrossRef]
- Caicedo Díaz, G.E.; Rozo Wilches, L.S.; Rengifo, G. La achira: Alternativa Agroindustrial Para Áreas de Economía Campesina. Produmedios 2003, 30. Available online: http://bibliotecadigital.agronet.gov.co/bitstream/11348/4071/1/La%20achira%20tecnicas%20de%20cultivo%20y%20beneficio.pdf. (accessed on 9 October 2020).
- Casierra-Posada, F.; Nieto, P.J.; Ulrichs, C. Growth, Production and Flower Quality in Calla Lily (Zantedeschia aethiopica (L.) K. Spreng) exposed to different light quality. Rev. UDCA Actual. Divulg. Científica 2012, 15, 97–105. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0123-42262012000100011 (accessed on 15 October 2020).
- Sánchez-Olivares, E.; Marín-Muñiz, J.L.; Hernandez-Alarcón, M.E. Radial oxygen loss by roots of native tropical wetland plants of Veracruz in response of different flooding conditions. Bot. Sci. 2019, 97, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Sandoval, L.; Zurita, F.; Ángel-Coronel, D.; Andrés, O.; Adame-García, J.; Marín-Muñíz, J.L. Influence of a new ornamental species (Spathiphyllum blandum) on the removal of cod, nitrogen, phosphorus and fecal coliforms: A mesocosm wetland study with pet and tezontle substrates. Water Sci. Technol. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandoval-Herazo, L.C.; Marín-Muñiz, J.L.; Orduñas, M.G.H.; Aleman-Chang, A.J. Role of Wetland Plants and Use of Ornamental Flowering Plants in Constructed Wetlands for Wastewater Treatment: A Review. Appl. Sci. 2019, 9, 685. [Google Scholar] [CrossRef] [Green Version]
- Vasconez, R.D.A.; Utreras, V.P.C.; Suquillo, I.D.V. Efecto Del Carbón Activado En La Germinación Y Brotación In Vitro De Citrus Limon (L.) Y Su Dinámica De Crecimiento. Bionatura 2018, 3. [Google Scholar] [CrossRef]
- Sandoval, L.; Marín-Muñiz, J.L.; Zamora-Castro, S.A.; Sandoval-Salas, F.; Alvarado-Lassman, A. Evaluation of Wastewater Treatment by Microcosms of Vertical Subsurface Wetlands in Partially Saturated Conditions Planted with Ornamental Plants and Filled with Mineral and Plastic Substrates. Int. J. Environ. Res. Public Health 2019, 16, 167. [Google Scholar] [CrossRef] [Green Version]
- Decezaro, S.T.; Wolff, D.B.; Pelissari, C.; Ramírez, R.J.; Formentini, T.A.; Goerck, J.; Sezerino, P.H. Influence of hydraulic loading rate and recirculation on oxygen transfer in a vertical flow constructed wetland. Sci. Total Environ. 2019, 668, 988–995. [Google Scholar] [CrossRef]
- Xiao, N.; Wu, R.; Huang, J.J.; Selvaganapathy, P.R. Development of a xurographically fabricated miniaturized low-cost, high-performance microbial fuel cell and its application for sensing biological oxygen demand. Sens. Actuators B Chem. 2020, 304, 127432. [Google Scholar] [CrossRef]
- Kumar, S.; Pratap, B.; Dubey, D.; Dutta, V. Microbial Communities in Constructed Wetland Microcosms and Their Role in Treatment of Domestic Wastewater. In Emerging Eco-friendly Green Technologies for Wastewater Treatment; Springer: Singapore, 2020; pp. 311–327. [Google Scholar] [CrossRef]
- Hussain, Z.; Arslan, M.; Malik, M.H.; Mohsin, M.; Iqbal, S.; Afzal, M. Treatment of the textile industry effluent in a pilot-scale vertical flow constructed wetland system augmented with bacterial endophytes. Sci. Total Environ. 2018, 645, 966–973. [Google Scholar] [CrossRef]
- Tulun, Ş. Treatment of Leachate Using Up-Flow Anaerobic Sludge Blanket Reactors/Vertical Flow Subsurface Constructed Wetlands. Ecol. Chem. Eng. S 2020, 27, 129–137. [Google Scholar] [CrossRef]
- Ashraf, S.; Afzal, M.; Rehman, K.; Tahseen, R.; Naveed, M.; Zahir, Z.A. Enhanced remediation of tannery effluent in constructed wetlands augmented with endophytic bacteria. Desalination Water Treat. 2018, 102, 93–100. [Google Scholar] [CrossRef]
- Lin, C.J.; Chyan, J.M.; Zhuang, W.X.; Vega, F.A.; Mendoza, R.M.O.; Senoro, D.B.; Liao, C.H. Application of an innovative front aeration and internal recirculation strategy to improve the removal of pollutants in subsurface flow constructed wetlands. J. Environ. Manag. 2020, 256, 109873. [Google Scholar] [CrossRef]
- Rodríguez-Momroy, J.; Durán-de-Bazúa, C. Remoción de Nitrógeno en un Sistema de Tratamiento de Aguas Residuales Usando Humedales Artificiales de Flujo Vertical a Escala de Banco. Tecnol. Cienc. Educ. 2006, 21, 25–33. Available online: https://www.redalyc.org/articulo.oa?id=48221104 (accessed on 25 October 2020).
- Rahimikhoob, H.; Sohrabi, T.; Delshad, M. Development of a Critical Nitrogen Dilution Curve for Basil (Ocimum basilicum L.) Under Greenhouse Conditions. J. Soil Sci. Plant Nutr. 2020, 1–11. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; García-González, M.C.; Ruelas-Monjardín, L.C.; Moreno-Casasola, P. Influence of different porous media and ornamental vegetation on wastewater pollutant removal in vertical subsurface flow wetland microcosms. Environ. Eng. Sci. 2018, 35, 88–94. [Google Scholar] [CrossRef]
- Casierra-Martínez, H.A.; Charris-Olmos, J.C.; Caselles-Osorio, A.; Parody-Muñoz, A.E. Organic matter and nutrients removal in tropical constructed wetlands using Cyperus ligularis (Cyperaceae) and Echinocloa colona (Poaceae). Water Air Soil Pollut. 2017, 228, 338. [Google Scholar] [CrossRef]
- Gadkari, S.; Shemfe, M.; Sadhukhan, J. Microbial fuel cells: A fast converging dynamic model for assessing system performance based on bioanode kinetics. Int. J. Hydrog. Energy 2019, 44, 15377–15386. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol 2008, 78, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Song, H.; Yan, X.; Chen, W.N. The effect of external resistance on biofilm formation and inte rnal resistance in Shewanella inoculated microbial fuel cells. RSC Adv. 2016, 6, 20317–20323. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Molognoni, D.; Puig, S.; Balaguer, M.D.; Colprim, J. Role of Operating Conditions on Energetic Pathways in a Microbial Fuel Cell. Energy Procedia 2015, 74, 728–735. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.V.; Raghavulu, S.V.; Peri, D.; Sarma, P.N. Integrated function of microbial fuel cell (MFC) as bio-electrochemical treatment system associated with bioelectricity generation under higher substrate load. Biosens. Bioelectron. 2009, 24, 2021–2027. [Google Scholar] [CrossRef]
- Walter, X.A.; Santoro, C.; Greenman, J.; Ieropoulos, I. Self-stratifying microbial fuel cell: The importance of the cathode electrode immersion height. Int. J. Hydrog. Energy 2019, 44, 4524–4532. [Google Scholar] [CrossRef]






| Parameter | Zantedeschia aethiopica | Canna hybrids |
|---|---|---|
| pH | ||
| Influent | 7.8 ± 0.4 | |
| Effluent | 7.5 ± 0.2 | 7.5 ± 0.2 |
| DO (mg/L) | ||
| Influent | 1.4 ± 0.3 | |
| Effluent | 7.1 ± 0.3 | 8.7 ± 0.4 |
| Water temperature (°C) | ||
| Influent | 7.8 ± 0.4 | |
| Effluent | 7.5 ± 0.2 | 7.5 ± 0.2 |
| BOD (mg/L) | ||
| Influent | 286 ± 28.4 | |
| Effluent | 8.6 ± 7.3 | 5.4 ± 3.1 |
| Removal (%) | 96.9 ± 2.5 | 98.1 ± 0.9 |
| TKN (mg/L) | ||
| Influent | 87.5 ± 16.8 | |
| Effluent | 32.8 ± 17.0 | 26.7 ± 11.3 |
| Removal (%) | 64.9 ± 12.7 | 69.49 ± 12.9 |
| P-PO4 (mg/L) | ||
| Influent | 7.9 ± 2.7 | |
| Effluent | 6.3 ± 1.2 | 5.8 ± 0.3 |
| Removal (%) | 20.2 ± 15.2 | 26.6 ± 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Moreno, H.R.; Marín-Muníz, J.L.; Sánchez-Dela-Cruz, E.; Nakase, C.; Del Ángel-Coronel, O.A.; Reyes-Gonzalez, D.; Nava-Valente, N.; Sandoval-Herazo, L.C. Bioelectricity Generation and Production of Ornamental Plants in Vertical Partially Saturated Constructed Wetlands. Water 2021, 13, 143. https://doi.org/10.3390/w13020143
González-Moreno HR, Marín-Muníz JL, Sánchez-Dela-Cruz E, Nakase C, Del Ángel-Coronel OA, Reyes-Gonzalez D, Nava-Valente N, Sandoval-Herazo LC. Bioelectricity Generation and Production of Ornamental Plants in Vertical Partially Saturated Constructed Wetlands. Water. 2021; 13(2):143. https://doi.org/10.3390/w13020143
Chicago/Turabian StyleGonzález-Moreno, Humberto Raymundo, José Luis Marín-Muníz, Eddy Sánchez-Dela-Cruz, Carlos Nakase, Oscar Andrés Del Ángel-Coronel, David Reyes-Gonzalez, Noemí Nava-Valente, and Luis Carlos Sandoval-Herazo. 2021. "Bioelectricity Generation and Production of Ornamental Plants in Vertical Partially Saturated Constructed Wetlands" Water 13, no. 2: 143. https://doi.org/10.3390/w13020143