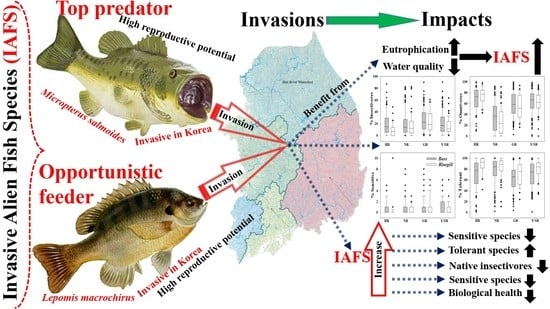
Relative Abundance and Invasion Dynamics of Alien Fish Species Linked to Chemical Conditions, Ecosystem Health, Native Fish Assemblage, and Stream Order
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Overview of the Selected Invasive Fish Species
2.1.1. Largemouth Bass
2.1.2. Bluegill
2.2. Study Area
2.3. Fish Sampling
2.4. Analysis of Ecological Indicators
2.5. IBI for Ecosystem Health Analyses
2.6. Physicochemical Analyses of Water Quality
2.7. Statistical Analyses
3. Results and Discussion
3.1. Ambient Water Chemistry and Invasive Species
3.2. Ecological Guild Dynamics and RA of Invasive Species
3.3. Ecosystem Health Dynamics and Invasive Species
3.4. Stream Order Preferences of Invasive Fish Species
3.5. Relationships between Trophic and Tolerance Guild and IAFS
4. Conclusions and Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piria, M.; Simonović, P.; Kalogianni, E.; Vardakas, L.; Koutsikos, N.; Zanella, D.; Ristovska, M.; Apostolou, A.; Adrović, A.; Mrdak, D.; et al. Alien freshwater fish species in the Balkans-Vectors and pathways of introduction. Fish Fish. 2018, 19, 138–169. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Kennard, M.J.; Liu, Y.; Sui, X.; Chen, Y.; Li, K.; Wang, G.; Chen, Y. Understanding invasion success of Pseudorasbora parva in the Qinghai-Tibetan Plateau: Insights from life-history and environmental filters. Sci. Total Environ. 2019, 694, 133739. [Google Scholar] [CrossRef] [PubMed]
- Lomnicky, G.A.; Whittier, T.R.; Hughes, R.M.; Peck, D.V. Distribution of nonnative aquatic vertebrates in western US streams and rivers. N. Am. J. Fish. Manag. 2007, 27, 1082–1093. [Google Scholar] [CrossRef]
- Cucherousset, J.; Olden, J.D. Ecological impacts of nonnative freshwater fishes. Fisheries 2011, 36, 215–230. [Google Scholar] [CrossRef]
- Casatti, L.; Teresa, F.B.; Zeni, J.d.O.; Ribeiro, M.D.; Brejão, G.L.; Ceneviva-Bastos, M. More of the same: High functional redundancy in stream fish assemblages from tropical agroecosystems. Environ. Manag. 2015, 55, 1300–1314. [Google Scholar] [CrossRef]
- Marquez, J.F.; Lee, A.M.; Aanes, S.; Engen, S.; Herfindal, I.; Salthaug, A.; Sæther, B. Spatial scaling of population synchrony in marine fish depends on their life history. Ecol. Lett. 2019, 13360. [Google Scholar] [CrossRef]
- Bae, M.-J.; Murphy, C.A.; García-Berthou, E. Temperature and hydrologic alteration predict the spread of invasive Largemouth Bass (Micropterus salmoides). Sci. Total Environ. 2018, 639, 58–66. [Google Scholar] [CrossRef]
- García-Berthou, E. The characteristics of invasive fishes: What has been learned so far? J. Fish. Biol. 2007, 71, 33–55. [Google Scholar] [CrossRef]
- Copp, G.H.; Vilizzi, L.; Wei, H.; Li, S.; Piria, M.; Al-Faisal, A.J.; Almeida, D.; Atique, U.; Al-Wazzan, Z.; Bakiu, R.; et al. Speaking their language—Development of a multilingual decision-support tool for communicating invasive species risks to decision makers and stakeholders. Environ. Model. Softw. 2021, 135, 104900. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Shackleton, C.M. The role of invasive alien species in shaping local livelihoods and human well-being: A review. J. Environ. Manag. 2019, 229, 145–157. [Google Scholar] [CrossRef]
- Atique, U.; Kwon, S.; An, K.-G. Linking weir imprints with riverine water chemistry, microhabitat alterations, fish assemblages, chlorophyll-nutrient dynamics, and ecological health assessments. Ecol. Indic. 2020, 117, 106652. [Google Scholar] [CrossRef]
- Hara, J.; Atique, U.; An, K.G. Multiyear links between water chemistry, algal chlorophyll, drought-flood regime, and nutrient enrichment in a morphologically complex reservoir. Int. J. Environ. Res. Public Health 2020, 17, 3139. [Google Scholar] [CrossRef]
- Atique, U.; An, K.-G. Reservoir water quality assessment based on chemical parameters and the chlorophyll dynamics in relation to nutrient regime. Pol. J. Environ. Stud. 2019, 28, 1043–1061. [Google Scholar] [CrossRef]
- Kim, J.-J.; Atique, U.; An, K.-G. Long-term ecological health assessment of a restored urban stream based on chemical water quality, physical habitat conditions and biological integrity. Water 2019, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.A.; Jewel, M.A.S.; Atique, U.; Paul, A.K.; Iqbal, S. Seasonal and spatial variation of flagellate communities in a tropical river. Limnologica 2020, 85, 125824. [Google Scholar] [CrossRef]
- Lee, M.; Jung, I. Assessment of an urban stream restoration project by cost-benefit analysis: The case of Cheonggyecheon stream in Seoul, South Korea. KSCE J. Civ. Eng. 2016, 20, 152–162. [Google Scholar] [CrossRef]
- Koutsikos, N.; Zogaris, S.; Vardakas, L.; Kalantzi, O.-I.; Dimitriou, E.; Economou, A.N. Tracking non-indigenous fishes in lotic ecosystems: Invasive patterns at different spatial scales in Greece. Sci. Total Environ. 2019, 659, 384–400. [Google Scholar] [CrossRef]
- Bae, D.-Y.; Atique, U.; Yoon, J.; Lim, B.; An, K.-G. Ecological risk assessment of urban streams using fish biomarkers of DNA damages and physiological responses. Pol. J. Environ. Stud. 2020, 29, 1–10. [Google Scholar] [CrossRef]
- Atique, U.; Iqbal, S.; Khan, N.; Qazi, B.; Javeed, A.; Anjum, K.M.; Haider, M.S.; Khan, T.A.; Mahmood, S.; Sherzada, S. Multivariate assessment of water chemistry and metals in a river impacted by tanning industry. Fresenius Environ. Bull. 2020, 29, 3013–3025. [Google Scholar]
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Qadir, A.; Malik, R.N. Assessment of an index of biological integrity (IBI) to quantify the quality of two tributaries of river Chenab, Sialkot, Pakistan. Hydrobiologia 2009, 621, 127–153. [Google Scholar] [CrossRef]
- An, K.-G.; Choi, J.W.; Lee, Y.J. Modifications of ecological trophic structures on chemical gradients in lotic ecosystems and their relations to stream ecosystem health. Anim. Cells Syst. 2013, 17, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Atique, U.; An, K.-G. Stream health evaluation using a combined approach of multi-metric chemical pollution. Water 2018, 10, 661. [Google Scholar] [CrossRef] [Green Version]
- Argillier, C.; Caussé, S.; Gevrey, M.; Pédron, S.; De Bortoli, J.; Brucet, S.; Emmrich, M.; Jeppesen, E.; Lauridsen, T.; Mehner, T.; et al. Development of a fish-based index to assess the eutrophication status of European lakes. Hydrobiologia 2013, 704, 193–211. [Google Scholar] [CrossRef] [Green Version]
- Blabolil, P.; Logez, M.; Ricard, D.; Prchalová, M.; Říha, M.; Sagouis, A.; Peterka, J.; Kubečka, J.; Argillier, C. An assessment of the ecological potential of Central and Western European reservoirs based on fish communities. Fish. Res. 2016, 173, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Domínguez, R.; MacI, S.; Courrat, A.; Lepage, M.; Borja, A.; Uriarte, A.; Neto, J.M.; Cabral, H.; Straykov, V.; Franco, A.; et al. Current developments on fish-based indices to assess ecological-quality status of estuaries and lagoons. Ecol. Indic. 2012, 23, 34–45. [Google Scholar] [CrossRef]
- Long, J.M.; Walker, D.J. Small scale application and assessment of an Index of Biotic Integrity for a large boreal river. Hydrobiologia 2005, 544, 177–187. [Google Scholar] [CrossRef]
- Moon, W.-K.; Atique, U.; An, K.-G. Ecological risk assessments and eco-toxicity analyses using chemical, biological, physiological responses, DNA damages and gene-level biomarkers in Zebrafish (Danio rerio) in an urban stream. Chemosphere 2020, 239, 124754. [Google Scholar] [CrossRef]
- Saeed, F.; Iqbal, K.J.; Atique, U.; Javid, A.; Khan, N.; Iqbal, S.; Majeed, H.; Azmat, H.; Khan, B.Y.A.; Baboo, I.; et al. Toxic trace metals assessment in selected organs of edible fish species, sediment and water in Head Punjnad, Punjab, Pakistan. Punjab Univ. J. Zool. 2020, 35, 43–50. [Google Scholar] [CrossRef]
- An, K.-G.; Choi, S.-S. An assessment of aquatic ecosystem health in a temperate watershed using the index of biological integrity. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2003, 36, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Khanom, D.A.; Nesa, A.; Jewel, M.A.S.; Haque, M.A.; Paul, A.K.; Iqbal, S.; Atique, U.; Alam, L. Muscular tissue bioaccumulation and health risk assessment of heavy metals in two edible fish species (Gudusia chapra and Eutropiichthys vacha) in Padma River, Bangladesh. Punjab Univ. J. Zool. 2020, 35, 81–89. [Google Scholar] [CrossRef]
- Iqbal, S.; Atique, U.; Mahboob, S.; Haider, M.S.; Iqbal, H.S.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Mughal, M.S. Effect of supplemental selenium in fish feed boosts growth and gut enzyme activity in juvenile tilapia (Oreochromis niloticus). J. King Saud Univ. Sci. 2020, 32, 2610–2616. [Google Scholar] [CrossRef]
- Diana, M.; Allan, J.D.; Infante, D. The influence of physical habitat and land use on stream fish assemblages in southeastern Michigan. Am. Fish. Soc. Symp. 2006, 48, 359–374. [Google Scholar]
- Crooks, J.A.; Chang, A.L.; Ruiz, G.M. Aquatic pollution increases the relative success of invasive species. Biol. Invasions 2011, 13, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Atique, U.; An, K.-G. Water quality and trophic state index analysis in relation to land use patterns in agricultural reservoirs. Korea Soc. Environ. Ecol. Proc. 2019, 2019, 110. [Google Scholar]
- Jang, M.-H.; Kim, J.-G.; Park, S.-B.; Jeong, K.-S.; Cho, G.-I.; Joo, G.-J. The current status of the distribution of introduced fish in large river systems of South Korea. Int. Rev. Hydrobiol. 2002, 87, 319–328. [Google Scholar] [CrossRef]
- Mamun, M.; Kim, S.; An, K.-G. Distribution pattern prediction of an invasive alien species largemouth bass using a maximum entropy model (MaxEnt) in the Korean peninsula. J. Asia Pac. Biodivers. 2018, 11, 516–524. [Google Scholar] [CrossRef]
- Kawamura, K.; Yonekura, R.; Katano, O.; Taniguchi, Y.; Saitoh, K. Origin and dispersal of bluegill sunfish, Lepomis macrochirus, in Japan and Korea. Mol. Ecol. 2006, 15, 613–621. [Google Scholar] [CrossRef]
- Kim, I.S. Freshwater fishes. Illustrated Encyclopedia of Fauna and Flora of Korea; Ministry of Education: Seoul, Korea, 1997; Volume 37.
- Kim, J.G.; Park, J.Y. Visual cells of the introduced bluegill Lepomis macrochirus (Pisces; Centropomidae) of Korea. Appl. Microsc. 2016, 46, 89–92. [Google Scholar] [CrossRef]
- US EPA. United States Environmental Protection Agency (US EPA). Fish. Field and Laboratory Methods for Evaluating the Biological Integrity of Surface Waters; EPA 600-R-92-111; Environmental Monitoring Systems Laboratory—Cincinnati Office of Modeling, Monitoring Systems, and Quality Assurance Office of Research Development; US EPA: Cincinnati, OH, USA, 1993; p. 348.
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; EPA 841-B-99-002; US Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999.
- An, K.-G.; Yeom, D.H.; Lee, S.K. Rapid bioassessments of kap stream using the index of biological integrity. Korean J. Environ. Biol. 2001, 19, 261–269. [Google Scholar]
- Choi, J.W.; Kumar, H.K.; Han, J.H.; An, K.-G. The development of a regional multimetric fish model based on biological integrity in lotic ecosystems and some factors influencing the stream health. Water Air Soil Pollut. 2011, 217, 3–24. [Google Scholar] [CrossRef]
- Lee, J.H.; An, K.G. Integrative restoration assessment of an urban stream using multiple modeling approaches with physical chemical, and biological integrity indicators. Ecol. Eng. 2014, 62, 153–167. [Google Scholar] [CrossRef]
- Jang, M.-H.; Joo, G.-J.; Lucas, M.C. Diet of introduced largemouth bass in Korean rivers and potential interactions with native fishes. Ecol. Freshw. Fish. 2006, 15, 315–320. [Google Scholar] [CrossRef]
- Brown, T.G.; Runciman, B.; Pollard, S.; Grant, A.D.A. Biological synopsis of largemouth bass (Micropterus salmoides). In Canadian Manuscript Report of Fisheries and Aquatic Sciences; Fisheries and Oceans Canada: Nanaimo, BC, Canada, 2009; p. 2884. [Google Scholar]
- Becker, G.C. Fishes of Wisconsin; The University of Wisconsin Press: Madison, WI, USA, 1983. [Google Scholar]
- Fuller, P.L.; Nico, L.G.; Williams, J.D. Nonindigenous Fishes Introduced into Inland Waters of the United States; Special Publication 27; American Fisheries Society: Bethesda, MD, USA, 1999. [Google Scholar]
- Azuma, M. Ecological release in feeding behavior: The case of bluegills in Japan. Hydrobiologia 1992, 243/244, 269–276. [Google Scholar] [CrossRef]
- Ministry of the Environment in Republic of Korea. The Outlaws of the Ecosystem—Invasive Alien Species. 2005. Available online: http://eng.me.go.kr/user/policies/6_nature_08.html (accessed on 26 March 2010).
- Froese, R.; Pauly, D. The Fishbase. 2005. Available online: www.fishbase.org (accessed on 4 April 2010).
- Strahler, A.N. Quantitative analysis of watershed geomorphology. Trans. Am. Geophys. Union 1957, 38, 913. [Google Scholar] [CrossRef] [Green Version]
- MOE/NIER. The Ministry of Environment/National Institute of Environmental Research (MOE/NIER). The Survey and Evaluation of Aquatic Ecosystem Health in Korea; The Ministry of Environment/National Institute of Environmental Research (NIER): Incheon, Korea, 2008.
- Kim, I.S.; Park, J.Y. Freshwater Fish of Korea; Kyohak Publishing: Seoul, Korea, 2002; p. 465. [Google Scholar]
- Nelson, J.S. Fishes of the World, 4th ed.; John Wiley & Sons: New York, NY, USA, 2006; p. 624. [Google Scholar]
- Sanders, R.E.; Milter, R.J.; Yondr, C.O.; Rankin, E.T. The use of external deformities, erosion, lesions, and tumors (DELT anormalies) in fish assemblages for characterising aquatic resources: A case study of seven Ohio streams. In Assessing the Sustainability and Biological Integrity of Water Resources Using Fish Communities; Simon, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 225–245. [Google Scholar]
- Karr, J.R.; Fausch, K.D.; Angermeier, P.L.; Yant, P.R.; Schlosser, I.J. Assessing biological integrity in running waters: A method and its rationale. Ill. Nat. Hist. Surv. Spec. Publ. 1986, 55, 28. [Google Scholar]
- Casatti, L.; Ferreira, C.P.; Langeani, F.A. Fish-based biotic integrity index for assessment of lowland streams in southeastern Brazil. Hydrobiologia 2009, 623, 173–189. [Google Scholar] [CrossRef]
- Rankin, E.T.; Yoder, C.O. Adjustments to the index of biotic integrity: A summary of Ohio experiences and some suggested modifications. In Assessing the Sustainability and Biological Integrity of Water Resources Using Fish Communities; Simon, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1999; p. 672. [Google Scholar]
- Crumpton, W.G.; Isenhart, T.M.; Mitchell, P.D. Nitrate and organic N analyses with second-derivative spectroscopy. Limnol. Oceanogr. 1992, 37, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Prepas, E.E.; Rigler, F.H. Improvements in quantifying the phosphorus concentration in lake water. Can. J. Fish. Aquat. Sci. 1982, 39, 822–829. [Google Scholar] [CrossRef]
- MOE (Ministry of Environment). Standard Methods for the Examination of Water Quality Contamination; The Ministry of Environments (MOE): Gwacheon, Korea, 2000.
- APHA (American Public Health Association); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater; AWWA: Washington, DC, USA, 2012; ISBN 9780875530130. [Google Scholar]
- Atique, U.; An, K.-G. Landscape heterogeneity impacts water chemistry, nutrient regime, organic matter and chlorophyll dynamics in agricultural reservoirs. Ecol. Indic. 2020, 110, 105813. [Google Scholar] [CrossRef]
- Lozon, J.D.; Macisaac, H.J. Biological invasions: Are they dependent on disturbance? Environ. Rev. 1997, 144, 131–144. [Google Scholar] [CrossRef]
- Paavola, M.; Olenin, S.; Leppäkoski, E. Are invasive species most successful in habitats of low native species richness across European brackish water seas? Estuar. Coast. Shelf Sci. 2005, 64, 738–750. [Google Scholar] [CrossRef]
- Anufriieva, E.V.; Shadrin, N.V. Extreme hydrological events destabilise aquatic ecosystems and open doors for alien species. Quat. Int. 2018, 475, 11–15. [Google Scholar] [CrossRef]
- Gallardo, B.; Aldridge, D.C. Inter-basin water transfers and the expansion of aquatic invasive species. Water Res. 2018, 143, 282–291. [Google Scholar] [CrossRef]
- Atique, U.; Byungjin, L.; Johee, Y.; An, K.-G. Biological health assessments of lotic waters by biotic integrity indices and their relations to water chemistry. Water 2019, 11, 436. [Google Scholar] [CrossRef] [Green Version]
- Villéger, S.; Miranda, J.R.; Hernández, D.F.; Mouillot, D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 2010, 20, 1512–1522. [Google Scholar] [CrossRef]
- Alexander, M.E.; Kaiser, H.; Weyl, O.L.F.; Dick, J.T.A. Habitat simplification increases the impact of a freshwater invasive fish. Environ. Biol. Fishes 2015, 98, 477–486. [Google Scholar] [CrossRef] [Green Version]
- Bunn, S.E.; Arthington, A.H. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ. Manag. 2002, 30, 492–507. [Google Scholar] [CrossRef] [Green Version]
- Castaldelli, G.; Pluchinotta, A.; Milardi, M.; Lanzoni, M.; Giari, L.; Rossi, R.; Fano, E.A. Introduction of exotic fish species and decline of native species in the lower Po basin, north-eastern Italy. Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 405–417. [Google Scholar] [CrossRef]
- Beard, Z.S.; Quist, M.C.; Hardy, R.S.; Ross, T.J. Patterns in fish assemblage structure in a small western stream. Copeia 2018, 106, 589–599. [Google Scholar] [CrossRef]
- Matthews, W.J. Patterns in Freshwater Ecology; Chapman and Hall: New York, NY, USA, 1998. [Google Scholar]
- Quist, M.C.; Hubert, W.A.; Isaak, D.J. Fish assemblage structure and relations with environmental conditions in a Rocky Mountain watershed. Can. J. Zool. 2004, 82, 1554–1565. [Google Scholar] [CrossRef] [Green Version]
- Rahel, F.J.; Hubert, W.A. Fish assemblages and habitat gradients in a Rocky Mountain—Great Plains stream: Biotic zonation and additive patterns of community change. Trans. Am. Fish. Soc. 1991, 120, 319–332. [Google Scholar] [CrossRef]
- Gard, R.; Flitner, G.A. Distribution and abundance of fishes in Sagehen Creek, California. J. Wildl. Manag. 1974, 38, 347–358. [Google Scholar] [CrossRef]
- Bonanno, G. Alien species: To remove or not to remove? That is the question. Environ. Sci. Policy 2016, 59, 67–73. [Google Scholar] [CrossRef]
- Hintz, W.D.; Schuler, M.S.; Jones, D.K.; Coldsnow, K.D.; Stoler, A.B.; Relyea, R.A. Nutrients influence the multi-trophic impacts of an invasive species unaffected by native competitors or predators. Sci. Total Environ. 2019, 694, 133704. [Google Scholar] [CrossRef]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Copp, G.H.; Robert Britton, J.; Cucherousset, J.; García-Berthou, E.; Kirk, R.; Peeler, E.; Stakėnas, S. Voracious invader or benign feline? A review of the environmental biology of European catfish Silurus glanis in its native and introduced ranges. Fish Fish. 2009, 10, 252–282. [Google Scholar] [CrossRef]
- Shrestha, B.B.; Shrestha, U.B.; Sharma, K.P.; Thapa-Parajuli, R.B.; Devkota, A.; Siwakoti, M. Community perception and prioritisation of invasive alien plants in Chitwan—Annapurna Landscape, Nepal. J. Environ. Manag. 2019, 229, 38–47. [Google Scholar] [CrossRef]






| Variables | Han River | Nakdong River | Geum River | Yeongsan/Seomjin Rivers |
|---|---|---|---|---|
| Geographic location coordinates | 36° N 30′, 126° E 24′–38° N 55′, 129° E 02′ | 35° N 03′, 127° E 27′–37° N 13′, 129° E 18′ | 35° N 35′, 126° E 41′–37° N 05′, 128° E 25′ | 34° N 40′, 126° E 26′–35° N 50′, 127° E 53′ |
| Human Population * | 27,432,203 | 13,726,794 | 6,251,437 | 4,174,595 |
| Daily discharge (m3/s) * | 1,089,801.92 | 1,363,452.72 | 692,017.98 | 235,607.66 |
| Ambient Factors | Largemouth Bass | Bluegill | t-Test | ||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD (Min-Max) | n | Mean ± SD (Min-Max) | F Value | t Value | p Value | |
| BOD (mg/L) | 532 | 2.63 ± 2.3 (0–16.7) | 178 | 2.91 ± 2.55 (0–17) | 0.806 | −1.419 | 0.156 |
| COD (mg/L) | 381 | 6.15 ± 2.9 (1–18) | 128 | 6.66 ± 3.03 (2–19) | 0.060 | −1.572 | 0.117 |
| TN (mg/L) | 525 | 2.64 ± 2.4 (0–20.00) | 175 | 2.95 ± 2.6 (0–17) | 2.923 | −1.479 | 0.088 |
| NO3-N (mg/L) | 526 | 1.50 ± 1.1 (0.02–5.96) | 178 | 1.66 ± 1.2 (0–6) | 2.591 | −1.592 | 0.112 |
| NH4-N (mg/L) | 422 | 0.40 ± 1.4 (0–7.09) | 137 | 0.62 ± 1.7 (0–5.7) | 4.787 | −1.493 | 0.136 |
| TP (µg/L) | 522 | 140 ± 170 (0–1080) | 177 | 160 ± 190 (0–1000) | 3.185 | −1.398 | 0.075 |
| PO4-P (mg/L) | 514 | 0.08 ± 0.12 (0–0.82) | 171 | 0.10 ± 0.15 (0–1) | 3.279 | −1.502 | 0.134 |
| TSS (mg/L) | 465 | 12.25 ± 12.6 (0–96) | 158 | 11.58 ± 10.69 (0–69) | 1.597 | 0.600 | 0.549 |
| Conductivity (µS/cm) | 381 | 318.9 ± 221.7 (58–1456) | 125 | 380.18 ± 260.05 (58–1279) | 8.062 | −2.565 | 0.011 |
| Chl-a (µg/L) | 527 | 16.7 ± 27.5 (0–252.3) | 175 | 19.43 ± 28.05 (0–154) | 1.285 | −1.131 | 0.259 |
| Ambient Factors | Criteria | Largemouth Bass RA (%) | Bluegill RA (%) |
|---|---|---|---|
| Ambient nutrients | TN (%) | 2.64 ± 2.367 (0–20) | 2.95 ± 2.585 (0–17) |
| TP (%) | 0.14 ± 0.17 (0–1.08) | 0.16 ± 0.19 (0–1) | |
| Chl-a (%) | 16.70 ± 27.36 (0–0.82) | 19.43 ± 28.05 (0–154) | |
| TN:TP Ratios (%) | TN:TP ≤ 100 | 95.3 | 96.0 |
| 100 < TN:TP < 200 | 3.7 | 3.4 | |
| TN:TP > 300 | 0 | 0 | |
| TN (%) | TN ≤ 1 | 14.8 | 15.5 |
| TN ≤ 2 | 36.1 | 28.2 | |
| TN > 3 | 25.5 | 31.0 | |
| TP (%) | TP ≤ 0.02 | 10.7 | 13.2 |
| TP ≤ 0.05 | 22.6 | 18.4 | |
| TP > 0.1 | 42.5 | 44.3 |
| Category | Metric | Scoring Criteria | Four Major Watersheds | |||||
|---|---|---|---|---|---|---|---|---|
| 5 | 3 | 1 | HR | NR | GR | YR | ||
| Species richness and compositions | M1: Total number of native fish species | Expectations of M1–M3 vary with stream size and region. | 3.25 | 2.14 | 3.62 | 3.25 | ||
| M2: Number of riffle benthic species | 2.49 | 1.23 | 1.73 | 1.29 | ||||
| M3: Number of sensitive species | 2.53 | 1.57 | 1.52 | 1.91 | ||||
| M4: Proportion of individuals as tolerant species | <5 | 5–20 | >20 | 2.2 | 1.85 | 1.3 | 1.52 | |
| Trophic compositions | M5: Proportion of individuals as omnivore species | <20 | 20–45 | >45 | 2.65 | 2.9 | 1.74 | 1.97 |
| M6: Proportion of individuals as native insectivore species | >45 | 45–20 | <20 | 3.45 | 2.94 | 2.69 | 3.06 | |
| Fish abundance and conditions | M7: Total number of native individuals | Expectations of M7 vary with stream size and region. | 2.6 | 1.6 | 3.09 | 2.2 | ||
| M8: Proportion of individuals with deformities, erosion, lesion, and tumors (DELT) | 0 | 0–1 | >1 | 4.22 | 4.65 | 4.35 | 4.23 | |
| Outcome | 23.39 (Fair) | 18.88 (Poor) | 20.04 (Fair) | 19.43 (Poor) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Atique, U.; An, K.-G. Relative Abundance and Invasion Dynamics of Alien Fish Species Linked to Chemical Conditions, Ecosystem Health, Native Fish Assemblage, and Stream Order. Water 2021, 13, 158. https://doi.org/10.3390/w13020158
Kim JY, Atique U, An K-G. Relative Abundance and Invasion Dynamics of Alien Fish Species Linked to Chemical Conditions, Ecosystem Health, Native Fish Assemblage, and Stream Order. Water. 2021; 13(2):158. https://doi.org/10.3390/w13020158
Chicago/Turabian StyleKim, Ji Yoon, Usman Atique, and Kwang-Guk An. 2021. "Relative Abundance and Invasion Dynamics of Alien Fish Species Linked to Chemical Conditions, Ecosystem Health, Native Fish Assemblage, and Stream Order" Water 13, no. 2: 158. https://doi.org/10.3390/w13020158