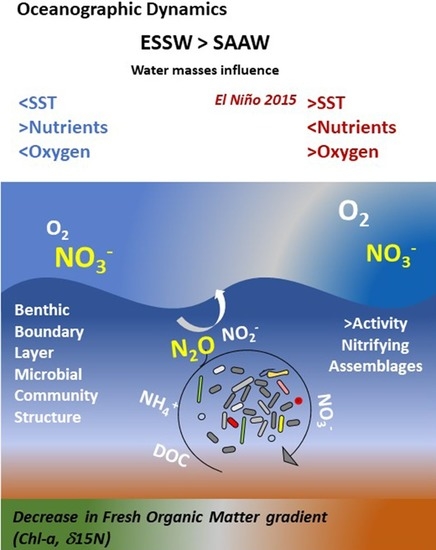
Biogeochemical Responses and Seasonal Dynamics of the Benthic Boundary Layer Microbial Communities during the El Niño 2015 in an Eastern Boundary Upwelling System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Column Chemical Analyses
2.2. Sediment Chemical Analyses
2.3. Microbial Communities’ Analyses
3. Results and Discussion
3.1. Oceanographic Features Observed
3.2. Biogeochemical Conditions in the Water Column
3.3. Sediment Conditions during the Selected Months
3.4. Microbial Community Dynamics in the Different Biomes
3.5. Nitrifying Microbial Groups and Their Predominance in the Bottom Water during Non-Upwelling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strub, P.T.; Mesías, I.; Montecino, V.; Rutllant, I.; Salinas, S. Coastal circulation off western South America. In The Sea; Robinson, A.R., Brink, K.H., Eds.; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Bertagnolli, A.D.; Stewart, F.J. Microbial niches in marine oxygen minimum zones. Nat. Rev. Microbiol. 2018, 16, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, M.; Farías, L.; Gallegos, M. Seasonal cycle of N2O vertical distribution and air–sea fluxes over the continental shelf waters off central Chile (∼36° S). Prog. Oceanogr. 2007, 75, 383–395. [Google Scholar] [CrossRef]
- Farías, L.; Besoain, V.; García, L.S. Presence of nitrous oxide hotspots in the coastal upwelling area off central Chile: An analysis of temporal variability based on ten years of a biogeochemical time series. Environ. Res. Lett. 2015, 10, 044017. [Google Scholar]
- Oerder, V.; Bento, J.P.; Morales, C.E.; Hormazabal, S.; Pizarro, O. Coastal Upwelling Front Detection off Central Chile (36.5–37° S) and Spatio-Temporal Variability of Frontal Characteristics. Remote Sens. 2018, 10, 690. [Google Scholar] [CrossRef] [Green Version]
- Fuenzalida, R.; Schneider, W.; Garcés-Vargas, J.; Bravo, L.; Lange, C. Vertical and horizontal extension of the oxygen minimum zone in the eastern South Pacific Ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 992–1003. [Google Scholar] [CrossRef]
- Ulloa, O.; Escribano, R.; Hormazabal, S.; Quiñones, R.A.; González, R.R.; Ramos, M. Evolution and biological effects of the 1997–98 El Niño in the upwelling ecosystem off northern Chile. Geophys. Res. Lett. 2001, 28, 1591–1594. [Google Scholar] [CrossRef]
- Santoso, A.; Phaden, M.J.; Cai, W. The Defining Characteristics of ENSO Extremes and the Strong 2015/2016 El Niño. Rev. Geophys. 2017, 55, 1079–1129. [Google Scholar] [CrossRef]
- Graco, M.; Correa, D.; García, W.; Sarmiento, M. Impactos del ENSO en la biogeoquímica del sistema de afloramiento frente a Perú central, febrero 2013-diciembre 2015. In Boletin Trimestral Oceanografico; Instituto del Mar del Perú: Callao District, Peru, 2016; Volume 2, pp. 2–6. [Google Scholar]
- Anabalón, V.; Morales, C.E.; González, H.E.; Menschel, E.; Schneider, W.; Hormazabal, S.; Valencia, L.; Escribano, R. Micro-phytoplankton community structure in the coastal upwelling zone off Concepción (central Chile): Annual and inter-annual fluctuations in a highly dynamic environment. Prog. Oceanogr. 2016, 149, 174–188. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, R.P.; Masotti, I. Inter-annual variability of oceanographic conditions and phytoplankton in Valparaíso Bay (~33°S), central Chile. Rev. Biol. Mar. Oceanogr. 2019, 54, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Iriarte, J.L.; González, H.E. Primary Productivity and Biomass of Size-Fractionated Phytoplankton in an Upwelling Area, Bahía de Mejillones (23° S, Chile), during and after the 1997–1998 El Niño. Investig. Mar. 2002, 30, 114–115. [Google Scholar] [CrossRef]
- Löscher, C.R.; Kock, A.; Könneke, M.; LaRoche, J.; Bange, H.W.; Schmitz, R.A. Production of oceanic nitrous oxide by ammonia-oxidizing archaea. Biogeosciences 2012, 9, 2419–2429. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.E.; Buchwald, C.; Knapp, A.N.; Berelson, W.M.; Capone, D.G.; Casciotti, K.L. Nitrification and nitrous oxide production in the offshore waters of the Eastern Tropical South Pacific. Earth Space Sci. Open Arch. 2020, 35, e2020GB006716. [Google Scholar] [CrossRef]
- Frey, C.; Bange, H.W.; Achterberg, E.P.; Jayakumar, A.; Löscher, C.R.; Arévalo-Martínez, D.L.; León-Palmero, E.; Sun, M.; Sun, X.; Xie, R.C.; et al. Regulation of nitrous oxide production in low-oxygen waters off the coast of Peru. Biogeosciences 2020, 17, 2263–2287. [Google Scholar] [CrossRef] [Green Version]
- Aldunate, M.; De la Iglesia, R.; Bertagnolli, A.D.; Ulloa, O. Oxygen modulates bacterial community composition in the coastal upwelling waters off central Chile. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 156, 68–79. [Google Scholar] [CrossRef]
- Galán, A.; Molina, V.; Belmar, L.; Ulloa, O. Temporal variability and phylogenetic characterization of planktonic anammox bacteria in the coastal upwelling ecosystem off central Chile. Prog. Oceanogr. 2012, 92, 110–120. [Google Scholar] [CrossRef]
- Molina, V.; Belmar, L.; Levipan, H.A.; Ramírez-Flandes, S.; Anguita, C.; Galán, A.; Montes, I.; Ulloa, O. Spatiotemporal Distribution of Key Pelagic Microbes in a Seasonal Oxygen-Deficient Coastal Upwelling System of the Eastern South Pacific Ocean. Front. Mar. Sci. 2020, 7, 795. [Google Scholar] [CrossRef]
- Neira, C.; Sellanes, J.; Soto, A.; Gutiérrez, D.; Gallardo, V.A. Meiofauna and sedimentary organic matter off Central Chile: Response to changes caused by the 1997–1998 El Niño. Oceanol. Acta 2001, 24, 313–328. [Google Scholar] [CrossRef] [Green Version]
- Sellanes, J.; Quiroga, E.; Neira, C.; Gutiérrez, D. Changes of macrobenthos composition under different ENSO cycle conditions on the continental shelf off central Chile. Cont. Shelf Res. 2007, 27, 1002–1016. [Google Scholar] [CrossRef]
- Schulz, H.N.; Strotmann, B.; Gallardo, V.A.; Jørgensen, B.B. Population study of the filamentous sulfur bacteria Thioploca spp. off the Bay of Concepción, Chile. Mar. Ecol. Prog. Ser. 2000, 200, 117–126. [Google Scholar] [CrossRef]
- Contreras, S.; Pantoja, S.; Neira, C.; Lange, C.B. Biogeochemistry of surface sediments off Concepción (∼36° S), Chile: El Niño vs. non-El Niño conditions. Prog. Oceanogr. 2007, 75, 576–585. [Google Scholar] [CrossRef]
- Srain, B.M.; Sobarzo, M.; Daneri, G.; González, H.E.; Testa, G.; Farías, L.; Schwarz, A.; Pérez, N.; Pantoja-Gutiérrez, S. Fermentation and Anaerobic Oxidation of Organic Carbon in the Oxygen Minimum Zone of the Upwelling Ecosystem Off Concepción, in Central Chile. Front. Mar. Sci. 2020, 7, 533. [Google Scholar] [CrossRef]
- Silva, N.; Rojas, N.; Fedele, A. Water masses in the Humboldt Current System: Properties, distribution, and the nitrate deficit as a chemical water mass tracer for Equatorial Subsurface Water off Chile. Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 1004–1020. [Google Scholar] [CrossRef]
- Soto, E.; Caballero, W.; Quiroga, E. Composition and vertical distribution of metazoan meiofauna assemblages on the continental shelf off central Chile. Lat. Am. J. Aquat. Res. 2015, 43, 922–935. [Google Scholar]
- Soto, E.; Quiroga, E.; Ganga, B.; Alarcón, G. Influence of organic matter inputs and grain size on soft-bottom macrobenthic biodiversity in the upwelling ecosystem of central Chile. Mar. Biodivers. 2017, 47, 433–450. [Google Scholar] [CrossRef]
- Carpenter, J.H. The accuracy of the Winkler method for dissolved oxygen analysis. Limnol. Oceanogr. 1965, 10, 135–140. [Google Scholar] [CrossRef]
- Holmes, R.M.; Aminot, A.; Krouel, R.; Hooker, B.A.; Peterson, B.J. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci. 1999, 56, 1801–1808. [Google Scholar] [CrossRef]
- Knap, A.; Michaels, A.; Dow, R.L.; Johnson, R.; Gundersen, K.; Sorensen, J.; Close, A.; Howse, F.; Hammer, M.; Bates, N.R.; et al. Bermuda Atlantic Times-series Studies Methods Manual (Version 3). In Bermuda Biological Station for Research; US JGOFS Inc.: Woods Hole, MA, USA, 1993; Volume 108. [Google Scholar]
- Atlas, E.L. A Practical Manual for Use of the Technicon AutoAnalyzer in Seawater Nutrient Analyses; Department of Oceanography, Technical Report; Oregon State University: Oregon, OR, USA, 1971; Volume 215. [Google Scholar]
- Holm-Hansen, O.; Lorenzen, C.J.; Holmes, R.W.; Strickland, J.D.H. Fluorometric Determination of Chlorophyll. ICES J. Mar. Sci. 1965, 30, 3–15. [Google Scholar] [CrossRef]
- McAuliffe, C. Gas Chromatographic Determination of Solutes by Multiple Phase Equilibrium. Chem. Technol. 1971, 1, 46–51. [Google Scholar]
- Byers, S.C.; Mills, E.L.; Stewart, P.L. A comparison of methods of determining organic carbon in marine sediments, with suggestions for a standard method. Hydrobiologia 1978, 58, 43–47. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Gallardo, V.A.; Mayor, S.; Neira, C.; Vásquez, C.; Sellanes, J.; Rivas, M.; Soto, A.; Carrasco, F.; Baltazar, M. Effects of dissolved oxygen and fresh organic matter on the bioturbation potential of macrofauna in sublittoral sediments off Central Chile during the 1997/1998 El Niño. Mar. Ecol. Prog. Ser. 2000, 202, 81–99. [Google Scholar] [CrossRef] [Green Version]
- Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 1969, 14, 454–458. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, R.J.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Gorley, R.N.; Clarke, R.K. PERMANOVA + for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008; p. 214. [Google Scholar]
- Andersen, K.S.; Kirkegaard, R.H.; Karst, S.M.; Albertsen, M. Ampvis2: An R package to analyse and visualise 16S rRNA amplicon data. bioRxiv 2018, 299537. [Google Scholar] [CrossRef] [Green Version]
- Francis, C.A.; Roberts, K.J.; Beman, J.M.; Santoro, A.E.; Oakley, B.B. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc. Natl. Acad. Sci. USA 2005, 102, 14683–14688. [Google Scholar] [CrossRef] [Green Version]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [Green Version]
- Levipan, H.A.; Molina, V.; Fernandez, C. Nitrospina-like bacteria are the main drivers of nitrite oxidation in the seasonal upwelling area of the Eastern South Pacific (Central Chile ~36° S). Environ. Microbiol. Rep. 2014, 6, 565–573. [Google Scholar] [CrossRef]
- Levipan, H.A.; Molina, V.; Anguita, C.; Rain-Franco, A.; Belmar, L.; Fernandez, C. Variability of nitrifying communities in surface coastal waters of the Eastern South Pacific (∼36° S). Environ. Microbiol. Rep. 2016, 8, 851–864. [Google Scholar] [CrossRef]
- Carvajal, M.; Contreras-López, M.; Winckler, P.; Sepúlveda, I. Meteotsunamis Occurring Along the Southwest Coast of South America During an Intense Storm. Pure Appl. Geophys. 2017, 174, 3313–3323. [Google Scholar] [CrossRef]
- Devol, A.H. Denitrification including Anammox. In Nitrogen in the Marine Environment; Capone, D.G., Bronk, D.A., Mulholland, M.R., Carpenter, E.J., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 263–301. [Google Scholar]
- Devol, A.H. Denitrification, Anammox, and N2 Production in Marine Sediments. Annu. Rev. Mar. Sci. 2015, 7, 403–423. [Google Scholar] [CrossRef] [PubMed]
- Farías, L.; Castro-González, M.; Cornejo, M.; Charpentier, J.; Faúndez, J.; Boontanon, N.; Yoshida, N. Denitrification and nitrous oxide cycling within the upper oxycline of the eastern tropical South Pacific oxygen minimum zone. Limnol. Oceanogr. 2009, 54, 132–144. [Google Scholar] [CrossRef]
- Galán, A.; Faúndez, J.; Thamdrup, B.; Santibañez, J.F.; Farías, L. Temporal dynamics of nitrogen loss in the coastal upwelling ecosystem off central Chile: Evidence of autotrophic denitrification through sulfide oxidation. Limnol. Oceanogr. 2014, 59, 1865–1878. [Google Scholar] [CrossRef]
- Lam, P.; Lavik, G.; Jensen, M.M.; van de Vossenberg, J.; Schmid, M.; Woebken, D.; Gutiérrez, D.; Amann, R.; Jetten, M.S.M.; Kuypers, M.M.M. Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc. Natl. Acad. Sci. USA 2009, 106, 4752–4757. [Google Scholar] [CrossRef] [Green Version]
- Codispoti, L.A. An oceanic fixed nitrogen sink exceeding 400 Tg N a−1 vs the concept of homeostasis in the fixed-nitrogen inventory. Biogeosciences 2007, 4, 233–253. [Google Scholar]
- Nevison, C.; Butler, J.H.; Elkins, J.W. Global distribution of N2O and the ΔN2O-AOU yield in the subsurface ocean. Glob. Biogeochem. Cycles 2003, 17, 1119. [Google Scholar] [CrossRef]
- Pastene, M.; Quiroga, E.; Hurtado, C.F. Stable isotopes and geochemical indicators in marine sediments as proxies for anthropogenic impact: A baseline for coastal environments of central Chile (33° S). Mar. Pollut. Bull. 2019, 142, 76–84. [Google Scholar] [CrossRef]
- Bui, V.H.; Bui, M.D.; Rutschmann, P. Advanced Numerical Modeling of Sediment Transport in Gravel-Bed Rivers. Water 2019, 11, 550. [Google Scholar]
- Pfannkuche, O.; Soltwedel, T. Pigments of sediment core M30/1_MC24, in supplement to: Pfannkuche, O.; Soltwedel, T: Small benthic size classes along the N.W. European Continental Margin: Spatial and temporal variability in activity and biomass. Prog. Oceanogr. 1998, 42, 189–207. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; Findlay, A.J.; Pellerin, A. The Biogeochemical Sulfur Cycle of Marine Sediments. Front. Microbiol. 2019, 10, 849. [Google Scholar]
- Pereira, O.; Hochart, C.; Auguet, J.C.; Debroas, D.; Galand, P.E. Genomic ecology of Marine Group II, the most common marine planktonic Archaea across the surface ocean. Microbiologyopen 2019, 8, e00852. [Google Scholar] [CrossRef] [PubMed]
- Teeling, H.; Fuchs, B.M.; Bennke, C.M.; Krüger, K.; Chafee, M.; Kappelmann, L.; Reintjes, G.; Waldmann, J.; Quast, C.; Glöckner, F.O.; et al. Recurring patterns in bacterioplankton dynamics during coastal spring algae blooms. Elife 2016, 5, e11888. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, J.A.; Cram, J.A.; Needham, D.M. Marine microbial community dynamics and their ecological interpretation. Nat. Rev. Microbiol. 2015, 13, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Parada, A.E.; Fuhrman, J.A. Marine archaeal dynamics and interactions with the microbial community over 5 years from surface to seafloor. ISME J. 2017, 11, 2510–2525. [Google Scholar] [CrossRef] [PubMed]
- Canfield, D.E.; Stweart, F.J.; Thamdrup, O.; De Brabandere, L.; Dalsgaard, T.; DeLong, E.G.; Revsbech, N.P.; Ulloa, O. A Cryptic Sulfur Cycle in Oxygen-Minimum–Zone Waters off the Chilean Coast. Science 2010, 330, 1375–1378. [Google Scholar] [CrossRef] [Green Version]
- Murillo, A.A.; Ramírez-Flandes, S.; DeLong, E.F.; Ulloa, O. Enhanced metabolic versatility of planktonic sulfur-oxidizing γ-proteobacteria in an oxygen-deficient coastal ecosystem. Front. Mar. Sci. 2014, 1, 18. [Google Scholar] [CrossRef] [Green Version]
- Dyksma, S.; Pjevac, P.; Ovaneson, K.; Mussmann, M. Evidence for H2 consumption by uncultured Desulfobacterales in coastal sediments. Environ. Microbiol. 2018, 20, 450–461. [Google Scholar] [CrossRef]
- Coskun, O.K.; Özen, V.; Wankel, S.D.; Orsi, W.D. Quantifying population-specific growth in benthic bacterial communities under low oxygen using H218O. ISME J. 2019, 13, 1546–1559. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-L.; Zhang, J.; Sun, Q.-L.; Lian, C.; Sun, L. A comparative study revealed first insights into the diversity and metabolisms of the microbial communities in the sediments of Pacmanus and Desmos hydrothermal fields. PLoS ONE 2017, 12, e0181048. [Google Scholar] [CrossRef] [Green Version]
- Reji, L.; Tolar, B.B.; Smith, J.M.; Chavez, F.P.; Francis, C.A. Differential co-occurrence relationships shaping ecotype diversification within Thaumarchaeota populations in the coastal ocean water column. ISME J. 2019, 13, 1144–1158. [Google Scholar] [CrossRef]
- Wilson, J.M.; Litvin, S.Y.; Beman, J.M. Microbial community networks associated with variations in community respiration rates during upwelling in nearshore Monterey Bay, California. Environ. Microbiol. Rep. 2018, 10, 272–282. [Google Scholar] [CrossRef]
- Bristow, L.A.; Dalsgaard, T.; Tiano, L.; Mills, D.B.; Bertagnolli, A.D.; Wright, J.J.; Hallam, S.J.; Ulloa, O.; Canfield, D.E.; Revsbech, N.P.; et al. Ammonium and nitrite oxidation at nanomolar oxygen concentrations in oxygen minimum zone waters. Proc. Natl. Acad. Sci. USA 2016, 113, 10601–10606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schratzberger, M.; Ingels, J. Meiofauna matters: The roles of meiofauna in benthic ecosystems. J. Exp. Mar. Biol. Ecol. 2018, 502, 12–25. [Google Scholar] [CrossRef]
- Nascimento, F.J.A.; Näslund, J.; Elmgren, R. Meiofauna enhances organic matter mineralization in soft sediment ecosystems. Limnol. Oceanogr. 2012, 57, 338–346. [Google Scholar] [CrossRef]
- Bonaglia, S.; Nascimento, F.J.A.; Bartoli, M.; Klawonn, I.; Bruchert, V. Meiofauna increases bacterial denitrification in marine sediments. Nat. Commun. 2014, 5, 5133. [Google Scholar] [CrossRef] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, V.; Cornejo-D’Ottone, M.; Soto, E.H.; Quiroga, E.; Alarcón, G.; Silva, D.; Acuña, C.; Silva, N. Biogeochemical Responses and Seasonal Dynamics of the Benthic Boundary Layer Microbial Communities during the El Niño 2015 in an Eastern Boundary Upwelling System. Water 2021, 13, 180. https://doi.org/10.3390/w13020180
Molina V, Cornejo-D’Ottone M, Soto EH, Quiroga E, Alarcón G, Silva D, Acuña C, Silva N. Biogeochemical Responses and Seasonal Dynamics of the Benthic Boundary Layer Microbial Communities during the El Niño 2015 in an Eastern Boundary Upwelling System. Water. 2021; 13(2):180. https://doi.org/10.3390/w13020180
Chicago/Turabian StyleMolina, Verónica, Marcela Cornejo-D’Ottone, Eulogio H. Soto, Eduardo Quiroga, Guillermo Alarcón, Daniela Silva, Carla Acuña, and Nelson Silva. 2021. "Biogeochemical Responses and Seasonal Dynamics of the Benthic Boundary Layer Microbial Communities during the El Niño 2015 in an Eastern Boundary Upwelling System" Water 13, no. 2: 180. https://doi.org/10.3390/w13020180