Network Model Analysis of Residual Chlorine to Reduce Disinfection Byproducts in Water Supply Systems in Yangon City, Myanmar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.1.1. Yangon City
2.1.2. Ngamoeyeik Water Treatment Plant (N-WTP)
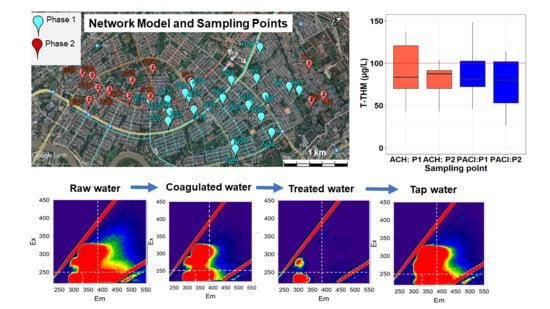
2.1.3. Service Area: North Okkalapa Township (N-OKP)
2.2. Field Sampling and Water Quality Analyses
2.3. 24-h Monitoring of Residual Chlorine in Water Distribution Network
2.4. Chlorine Decay Tests
2.5. Trihalomethane Formation Potential of Water Samples in the Water Treatment Plant
2.6. Data Analysis
2.7. Water Quality Simulation in the Networks
2.7.1. EPANET Network Model
2.7.2. Water Demand Allocation to Nodes
2.7.3. Water Loss
2.7.4. Reaction Coefficients
Bulk Decay Coefficient (Kb)
Wall Decay Coefficient (Kw)
THM formation coefficient (k’)
3. Results
3.1. Physical Parameters
3.2. Chemical Parameters
3.2.1. Ammonia Nitrogen
3.2.2. Residual Chlorine
3.2.3. Dissolved Organic Carbon (DOC)
3.2.4. UV254 Absorbance
3.2.5. Specific Ultraviolet Absorbance (SUVA)
3.2.6. Excitation Emission Matrix (EEM)
3.2.7. Trihalomethanes
3.2.8. Trihalomethane Formation Potential (THMFP)
3.2.9. 24-h Monitoring of Residual Chorine in the Networks
3.3. Microbial Parameters
3.4. Water Quality Simulation in the Water Distribution Network
3.4.1. Residual Chlorine (Phase 1)
3.4.2. Trihalomethanes (Phase 1)
3.4.3. Residual Chlorine (Phase 2)
3.4.4. Trihalomethanes (Phase 2)
4. Discussion
4.1. Effect of Changing Coagulant
4.2. Water Quality Comparison between Phase 1 and Phase 2
4.3. Effect of Organic Matter on Chlorine Decay and THM Formation
4.4. Water Distribution Network Modelling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Parameters | Equipment |
|---|---|---|
| 1 | pH, Temperature | LAQUAact, Horiba, Kyoto, Japan |
| 2 | Turbidity | Thermo Scientific TN-100, Thermo Scientific Eutech Instruments Pre Ltd., Singapore |
| 3 | Residual chlorine | DR 890 HACH Potable Colorimeter, HACH, CO, USA |
| 4 | Ammonia- nitrogen | |
| 5 | Trihalomethanes (Chloroform) | DR 6000 HACH Spectrophotometer, CO, USA |
| 6 | Trihalomethanes | Shimadzu GC-2010 Plus, Kyoto, Japan |
| 7 | UV254 Absorbance | UH-5300, Hitachi High-Tech Science Co., Tokyo, Japan |
| 8 | Dissolved Organic Carbon | TOC-L analyzer, Shimadzu, Kyoto, Japan |
| 9 | Excitation Emission Matrix (EEM) | Agilent Cray Eclipse Fluorescence Spectrophotometer, CA, USA |
| 10 | Bromide | Metrohm 861 Advance Compact IC, Metrohm, Herisau, Switzerland |
Appendix B
Appendix B.1. Water Demand Pattern

Appendix B.2. Batch Decay Tests of Chlorine

Appendix C

| Phase 1 | Phase2 | |||||
|---|---|---|---|---|---|---|
| ACH: P1 (n = 15) | PACl:P1 (n = 24) | Computed (n = 99) | ACH: P2 (n = 15) | PACl:P2 (n = 15) | Computed (n = 33) | |
| Standard deviation (SD) | 35 | 27 | 0 | 25 | 34 | 1 |
| Mean | 99 | 88 | 79 | 99 | 82 | 84 |
| Minimum | 43 | 43 | 78 | 66 | 26 | 82 |
| Maximum | 167 | 169 | 79 | 148 | 151 | 86 |
Appendix D






References
- Nieuwenhuijsen, M.; Toledano, M.; Eaton, N.; Fawell, J.; Elliott, P. Chlorination disinfection byproducts in water and their association with adverse reproductive outcomes. Occup. Environ. Med. 2000, 57, 73–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafa, N.; Matta, M.; Abdel-Halim, H. Simulation of Chlorine Decay in Water Distribution Networks Using EPANET–Case Study. Civ. Environ. Res. 2013, 3, 100–116. [Google Scholar]
- Jonkergouw, P.M.R.; Khu, S.-T.; Savic, D.A.; Zhong, D.; Hou, X.Q.; Zhao, H.-B. A Variable Rate Coefficient Chlorine Decay Model. Environ. Sci. Technol. 2009, 43, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Sivagansen, M. Predicting Chlorine Residuals and the Formation of T-THM in Drinking Water. J. Environ. Eng. 1998, 124, 1203–1210. [Google Scholar] [CrossRef]
- Gang, D.C.; Clevenger, T.E.; Banerji, S.K. Modeling Chlorine Decay in Surface Water. J. Environ. Inform. 2015, 1, 21–27. [Google Scholar] [CrossRef]
- WHO. World Health Organization, Guidelines for Drinking Water Quality, 3rd ed.; WHO: Geneva, Switzerland, 2008; Volume 1, pp. 451–454. Available online: https://www.who.int/water_sanitation_health/dwq/fulltext.pdf (accessed on 2 July 2021).
- Richardson, S.; Plewa, M.; Wagner, E.; Schoeny, R.; Demarini, D. Occurrence, Genotoxicity, and Carcinogenicity of Regulated and Emerging Disinfection Byproducts in Drinking Water: A Review and Roadmap for Research. Mutat. Res. Rev. Mutat. Res. 2007, 636, 178–242. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Water Treatment Manual: Disinfection. 2011. Available online: https://www.epa.ie/publications/compliance--enforcement/drinking-water/advice--guidance/Disinfection2_web.pdf (accessed on 28 April 2021).
- Ministry of Health Labour and Welfare (MHLW), Japan, Drinking Water Quality Standard. 2012. Available online: https://www.mhlw.go.jp/english/policy/health/water_supply/dl/4a.pdf (accessed on 15 May 2021).
- Japan Water Works Association (JWWA). Water Treatment Facilities of the Design Criteria for Water Supply Facilities. 2012. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000103931.pdf (accessed on 14 April 2021).
- Sakai, H.; Tokuhara, S.; Murakami, M.; Kosaka, K.; Oguma, K.; Takizawa, S. Comparison of Chlorination and Chloramination in Carbonaceous and Nitrogenous Disinfection Byproduct Formation Potentials with Prolonged Contact Time. Water Res. 2016, 88, 661–670. [Google Scholar] [CrossRef]
- Rakruam, P.; Wattanachira, S. Reduction of DOM Fractions and Their Trihalomethane Formation Potential in Surface River Water by In-Line Coagulation with Ceramic Membrane Filtration. J. Environ. Sci. 2014, 26, 529–536. [Google Scholar] [CrossRef]
- Musikavong, C.; Wattanachira, S. Identification of Dissolved Organic Matter in Raw Water Supply from Reservoirs and Canals as Precursors to Trihalomethanes Formation. J. Environ. Sci. Health Part A 2013, 48, 760–771. [Google Scholar] [CrossRef]
- Lundqvist, J.; Andersson, A.; Johannisson, A.; Lavonen, E.; Mandava, G.; Kylin, H.; Bastviken, D.; Oskarsson, A. Innovative Drinking Water Treatment Techniques Reduce the Disinfection-Induced Oxidative Stress and Genotoxic Activity. Water Res. 2019, 155, 182–192. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiao, F.; Wang, D.; Yan, M.; Bi, Z. Disinfection Byproduct Precursor Removal by Enhanced Coagulation and Their Distribution in Chemical Fractions. J. Environ. Sci. 2013, 25, 2207–2213. [Google Scholar] [CrossRef]
- Amarasooriya, A.A.G.D.; Weragoda, S.; Makehelwala, M.; Weerasooriya, R. Occurrence of Trihalomethane in Relation to Treatment Technologies and Water Quality under Tropical Conditions. H2Open J. 2018, 1. [Google Scholar] [CrossRef] [Green Version]
- Valdivia-Garcia, M.; Weir, P.; Graham, D.; Werner, D. Predicted Impact of Climate Change on Trihalomethanes Formation in Drinking Water Treatment. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Delpla, I.; Jung, A.-V.; Baurès, E.; Clement, M.; Thomas, O. Impacts of Climate Change on Surface Water Quality in Relation to Drinking Water Production. Environ. Int. 2009, 35, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Phetrak, A.; Lohwacharin, J.; Takizawa, S. Analysis of Trihalomethane Precursor Removal from Sub-Tropical Reservoir Waters by a Magnetic Ion Exchange Resin Using a Combined Method of Chloride Concentration Variation and Surrogate Organic Molecules. Sci. Total Environ. 2016, 539, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Osawa, H.; Lohwacharin, J.; Takizawa, S. Controlling Disinfection By-Products and Organic Fouling by Integrated Ferrihydrite–Microfiltration Process for Surface Water Treatment. Sep. Purif. Technol. 2017, 176, 184–192. [Google Scholar] [CrossRef]
- Momba, M.; Kfir, R.; Venter, S.; Cloete, T. An Overview of Biofilm Formation in Distribution Systems and Its Impact on the Deterioration of Water Quality. Water SA 2000, 26, 59–66. Available online: http://hdl.handle.net/10204/742 (accessed on 28 April 2021).
- Ding, S.; Deng, Y.; Bond, T.; Fang, C.; Cao, Z.; Chu, W. Disinfection Byproduct Formation during Drinking Water Treatment and Distribution: A Review of Unintended Effects of Engineering Agents and Materials. Water Res. 2019, 160, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Knibbe, W.-J.; Feng, C.; Liu, W.; Medema, G.; van der Meer, W. Potential Impacts of Changing Supply-Water Quality on Drinking Water Distribution: A Review. Water Res. 2017, 116, 135–148. [Google Scholar] [CrossRef]
- Karaderek, I.E.; Kara, S.; Muhammetoglu, A.; Muhammetoglu, H.; Soyupak, S. Management of chlorine dosing rates in urban water distribution networks using online continuous monitoring and modeling. Urban Water J. 2016, 13, 245–359. [Google Scholar] [CrossRef]
- JICA, A Strategic Urban Development Plan of Greater Yangon Final Report 1, April 2013. Available online: https://openjicareport.jica.go.jp/pdf/12122511.pdf (accessed on 19 March 2021).
- AWWA, Water Treatment Plant Design, Fourth Edition. 2004. Available online: https://www.academia.edu/25857768/Water_Treatment_Plant_Design_4th_Edition (accessed on 25 May 2021).
- Yangon City Development Committee. Research Report of Filter Improvement Task Force Team; Yangon City Development Committee (YCDC): Yangon, Myanmar, 2019.
- Ministry of Labour, Immigration and Population, The 2014 Myanmar Population and Housing Census, Yangon Region, Eastern District, North Okkalapa Township Report, October 2017. Available online: https://themimu.info/sites/themimu.info/files/documents/TspProfiles_Census_NorthOkkalapa_2014_ENG.pdf (accessed on 25 September 2020).
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Waste Water, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; pp. 5–70. [Google Scholar]
- Sorlini, S.; Biasibetti, M.; Gialdini, F.; Muraca, A. Modeling and Analysis of Chlorine Dioxide, Chlorite, and Chlorate Propagation in a Drinking Water Distribution System. J. Water Supply Res. Technol.-Aqua 2016, 65, 597–611. [Google Scholar] [CrossRef]
- Viessman, W. The Water Supply and Pollution Control, 8th ed.; Pearson: New York, NY, USA, 2008; pp. 126–191. [Google Scholar]
- Islam, M.S. Leakage Analysis and Management in the Water Distribution Network in a Selected Area of Bangkok. Master’s Thesis, Asian Institute of Technology, Khlong Nueng, Thailand, 2005. [Google Scholar]
- Rossman, L.A. EPANET 2 User’s Manual. 2000. Available online: https://www.microimages.com/documentation/Tutorials/Epanet2UserManual.pdf (accessed on 27 April 2021).
- Zhao, H.; Hu, C.; Liu, H.; Zhao, X.; Qu, J. Role of Aluminum Speciation in the Removal of Disinfection Byproduct Precursors by a Coagulation Process. Environ. Sci. Technol. 2008, 42, 5752–5758. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Tornyeviadzi, H.M.; Seidu, R. Modelling the Impact of Water Temperature, Pipe, and Hydraulic Conditions on Water Quality in Water Distribution Networks. Water Pract. Technol. 2021, 16, 387–403. [Google Scholar] [CrossRef]
- Clark, R.; Lykins, B.; Block, J.C.; Wymer, L.; Reasoner, D. Water Quality Changes in a simulated distribution system. J. Water Supply: Res. Technol.-Aqua 1994, 43, 263–277. [Google Scholar] [CrossRef]
- Chen, J.; Gu, B.; Leboeuf, E.; Pan, H.; Dai, S. Spectroscopic Characterization of Structural and Functional Properties of Natural Organic Matter Fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef]
- Jung, C.-W.; Son, H.-J. The Relationship between Disinfection By-Products Formation and Characteristics of Natural Organic Matter in Raw Water. Korean J. Chem. Eng. 2008, 25, 714–720. [Google Scholar] [CrossRef]
- Singer, P.C. Humic Substances as Precursors for Potentially Harmful Disinfection By-Products. Water Sci. Technol. 1999, 40, 25–30. [Google Scholar] [CrossRef]
- Hasan, A.; Thacker, N.P.; Bassin, J. Trihalomethane Formation Potential in Treated Water Supplies in Urban Metro City. Env. Monit Assess 2010, 168, 489–497. [Google Scholar] [CrossRef]
- Roccaro, P.; Chang, H.-S.; Vagliasindi, F.G.A.; Korshin, G.V. Differential Absorbance Study of Effects of Temperature on Chlorine Consumption and Formation of Disinfection By-Products in Chlorinated Water. Water Res. 2008, 42, 1879–1888. [Google Scholar] [CrossRef]
- Gilca, F.A.; Teodosiu, C.; Fiore, S.; Musteret, C. Emerging Disinfection Byproducts: A Review on Their Occurrence and Control in Drinking Water Treatment Processes. Chemosphere 2020, 259, 127476. [Google Scholar] [CrossRef]
- Kumar, A.; Bharanidharan, B.; Kumar, K.; Matial, N.; Dey, E.; Singh, M.; Thakur, V.; Sharma, S.; Malhotra, N. Design of Water Distribution System Using EPANET. Int. J. Adv. Res. 2015, 3, 789–812. [Google Scholar]



















| Filter Media | Filter Media Thickness | |
|---|---|---|
| Phase 1 | Phase 2 | |
| Anthracite | 45 cm | 30 cm |
| Sand | 30 cm | 90 cm |
| Gravel | 38 cm | 45 cm |
| Filtration Rate (m/Day) | No. of Basin | Remark | |
|---|---|---|---|
| Phase 1 | 220 (212) | 28 | dual media |
| Phase 2 | 190 (185) | 32 | dual media |
| JWWA design manual | 200–360 | more than 2 | dual media |
| AWWA design manual | 240–360 | dual media |
| No. | Parameters | Monitoring Methods | Sampling Methods and Analysis Site |
|---|---|---|---|
| 1 | pH, Temperature | Electrometric | Onsite |
| 2 | Turbidity | Nephelometric | |
| 3 | Residual chlorine | DPD | |
| 4 | Ammonia- nitrogen | Salicylate | Collected without head space in chorine-demand-free bottles/ YCDC Laboratory |
| 5 | Trihalomethanes (Chloroform) | THM plus (600 ppb) | |
| 6 | Trihalomethanes | Headspace GC-ECD | Filtrated with 0.45µm PTFE filter/ The University of Tokyo Laboratory (UT Lab) |
| 7 | UV254 Absorbance | Double beam Spectrophotometry | |
| 8 | Dissolved Organic Carbon | TC-IC | |
| 9 | Excitation Emission Matrix (EEM) | Absorbance Ex: 220–450 nm Em: 230–650 nm 5 nm steps | |
| 10 | Bromide | Ion Chromatography | |
| 11 | E. coli/ Total coliform | Membrane filtration method (Method 10029, USEPA) | Collected with sterilized plastic bags and chlorine quenching/YCDC Laboratory |
| Samples | Chlorine Dosage (mg/L) |
|---|---|
| N-WTP raw | 4.0 |
| Coagulated sample | 4.0 |
| Phase 1 treated | 2.0 |
| Phase 2 treated | 2.0 |
| Location | E. coli (CFU/100 mL) | Total Coliform (CFU/100 mL) |
|---|---|---|
| N-WTP raw water (n = 1) | 2400 | 2500 |
| N-WTP Treated water (Phase 1) (n = 1) | ND 1 | ND 1 |
| N-WTP Treated water (Phase 2) (n = 1) | ND 1 | ND 1 |
| N-OKP Tap water (n = 39) | ND 1 | ND 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zin, N.N.; Kazama, S.; Takizawa, S. Network Model Analysis of Residual Chlorine to Reduce Disinfection Byproducts in Water Supply Systems in Yangon City, Myanmar. Water 2021, 13, 2921. https://doi.org/10.3390/w13202921
Zin NN, Kazama S, Takizawa S. Network Model Analysis of Residual Chlorine to Reduce Disinfection Byproducts in Water Supply Systems in Yangon City, Myanmar. Water. 2021; 13(20):2921. https://doi.org/10.3390/w13202921
Chicago/Turabian StyleZin, Nwe Nwe, Shinobu Kazama, and Satoshi Takizawa. 2021. "Network Model Analysis of Residual Chlorine to Reduce Disinfection Byproducts in Water Supply Systems in Yangon City, Myanmar" Water 13, no. 20: 2921. https://doi.org/10.3390/w13202921