Neural Network and Random Forest-Based Analyses of the Performance of Community Drinking Water Arsenic Treatment Plants
Abstract
:1. Introduction
2. Overview of Field-Scale Technologies Adopted Globally
2.1. Oxidation
2.2. Adsorption
2.3. Filtration
2.4. Coagulation and Co-precipitation
2.5. Membrane-Based Technologies
2.6. Electrocoagulation
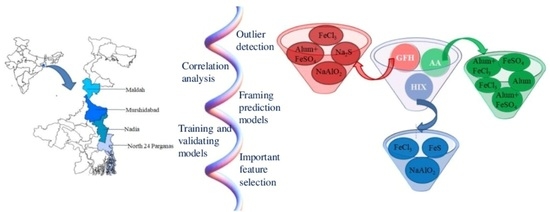
3. Methodology
3.1. Overview of AIRP Schemes in the Study Area
- Current economic indicators.
- The year-on-year increase in cumulative capacities till the current year.
- The cumulative capacities of arsenic-free water across the study area.
- Primary oxidation and disinfection methods.
- Cost of arsenic-free water production depending on technology and plant capacity.
3.2. Multivariate Modeling of AIRP Performance and Cost
- Organizing the collected database in terms of independent variables as significant technological and water quality parameters concerning the cost indicator and plant performance as responses.
- Data screening by outlier detection using the R package (‘OutlierDetection’).
- Correlation study for all the variables in the screened dataset using the R language.
- Development of a robust prediction model from the screened dataset through ANN in a python environment. Here, the utilization of the dataset concerning the formation of a predictive model consisted of two perspectives. First, the model preparation and calibration (training, testing, and cross-validation) were conducted from 90% of the screened data. However, around 10% of the total screened data were kept separate as new data for validating the predictive model. This approach of validation ensures the applicability of the developed model in any field condition of different scenarios, which was established with a separate dataset, as mentioned above.
- An RF-based classification algorithm was applied to the entire screened data in a python environment to identify the major influential parameters of the performance indicators.
4. Results and Discussion
4.1. AIRP Capacity by Region
4.2. Implemented Models for AIRP Projects
4.3. Current Field Scale Arsenic Removal Technologies
4.4. Economic Indicators in Current AIRPs
4.5. Safety and Testing Status of AIRPs
4.6. Benchmarking Presents Arsenic Management with Global Scenario
4.7. Prediction Models and their Applicability
4.7.1. ANN Study
4.7.2. Important Feature Selection by RF
4.7.3. Applicability of Machine Learning Based Framework
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.V.N.; Manan, F.A. Arsenic Removal Technologies and Future Trends: A Mini-Review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-jones, E. Introduction to Arsenic Contamination and Health Risk Assessment with Special Reference to Bangladesh. Rev. Environ. Contam. 2008, 197, 1–15. [Google Scholar] [CrossRef]
- Yadav, M.K.; Saidulu, D.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. Status and Management of Arsenic Pollution in Groundwater: A Comprehensive Appraisal of Recent Global Scenario, Human Health Impacts, Sustainable Field-Scale Treatment Technologies. J. Environ. Chem. Eng. 2021, 9, 105203. [Google Scholar] [CrossRef]
- Kumar, A.; Roy, M.B.; Roy, P.K.; Wallace, J.M. Assessment of Arsenic Removal Units in Arsenic-Prone Rural Area in Uttar Pradesh, India. J. Inst. Eng. Ser. A 2019, 100, 253–259. [Google Scholar] [CrossRef]
- Mukherjee, A.; Fryar, A.E.; Scanlon, B.R.; Bhattacharya, P.; Bhattacharya, A. Elevated Arsenic in Deeper Groundwater of the Western Bengal Basin, India: Extent and Controls from Regional to Local Scale. Appl. Geochem. 2011, 26, 600–613. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, S.; Chakraborty, M.; Duttagupta, S.; Bhattacharya, A.; Saha, D.; Bhattacharya, P.; Mitra, A.; Gupta, S. Occurrence, Predictors and Hazards of Elevated Groundwater Arsenic across India through Field Observations and Regional-Scale AI-Based Modeling. Sci. Total Environ. 2021, 759, 143511. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Sarkar, S.; Mukherjee, A.; Shamsudduha, M.; Ahmed, K.M.; Bhattacharya, A.; Mitra, A. Modeling Regional-Scale Groundwater Arsenic Hazard in the Transboundary Ganges River Delta, India and Bangladesh: Infusing Physically-Based Model with Machine Learning. Sci. Total Environ. 2020, 748, 141107. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S. Arsenic: Chemistry, Occurrence, and Exposure. In Handbook Arsenic Toxicology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1–49. ISBN 9780124199552. [Google Scholar]
- Bhakta, J.N.; Rana, S.; Jana, J.; Bag, S.K.; Lahiri, S.; Jana, B.B.; Panning, F.; Fechter, L. Current Status of Arsenic Contamination in Drinking Water and Treatment Practice in Some Rural Areas of West Bengal, India. J. Water Chem. Technol. 2016, 38, 366–373. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guidelines for Drinking-Water Quality: Incorporating the First Addendum; World Health Organization: Geneva, Switzerland, 2017; ISBN 9789241549950. [Google Scholar]
- Luong, V.T.; CañasKurz, E.E.; Hellriegel, U.; Luu, T.L.; Hoinkis, J.; Bundschuh, J. Iron-Based Subsurface Arsenic Removal Technologies by Aeration: A Review of the Current State and Future Prospects. Water Res. 2018, 133, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Palani, S.; Liong, S.Y.; Tkalich, P. An ANN Application for Water Quality Forecasting. Mar. Pollut. Bull. 2008, 56, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Antar, M.A.; Elassiouti, I.; Allam, M.N. Rainfall-Runoff Modelling Using Artificial Neural Networks Technique: A Blue Nile Catchment Case Study. Hydrol. Process. 2006, 20, 1201–1216. [Google Scholar] [CrossRef]
- Ozel, H.U.; Gemici, B.T.; Gemici, E.; Ozel, H.B.; Cetin, M.; Sevik, H. Application of Artificial Neural Networks to Predict the Heavy Metal Contamination in the Bartin River. Environ. Sci. Pollut. Res. 2020, 27, 42495–42512. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, P.S.; Kattil, K.V.; Yadav, M.K.; Gupta, A.K. Adsorptive Removal of Arsenic by Novel Iron/Olivine Composite: Insights into Preparation and Adsorption Process by Response Surface Methodology and Artificial Neural Network. J. Environ. Manag. 2018, 209, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Azqhandi, M.H.A.; Ghaedi, M.; Yousefi, F.; Jamshidi, M. Application of Random Forest, Radial Basis Function Neural Networks and Central Composite Design for Modeling and/or Optimization of the Ultrasonic Assisted Adsorption of Brilliant Green on ZnS-NP-AC. J. Colloid Interface Sci. 2017, 505, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Lovatti, B.P.O.; Nascimento, M.H.C.; Neto, Á.C.; Castro, E.V.R.; Filgueiras, P.R. Use of Random Forest in the Identification of Important Variables. MicroChem. J. 2019, 145, 1129–1134. [Google Scholar] [CrossRef]
- Ghaedi, M.; Ghaedi, A.M.; Negintaji, E.; Ansari, A.; Vafaei, A.; Rajabi, M. Journal of Industrial and Engineering Chemistry Random Forest Model for Removal of Bromophenol Blue Using Activated Carbon Obtained from AstragalusBisulcatus Tree. J. Ind. Eng. Chem. 2014, 20, 1793–1803. [Google Scholar] [CrossRef]
- Uddameri, V.; Silva, A.L.B.; Singaraju, S.; Mohammadi, G.; Hernandez, E.A. Tree-Based Modeling Methods to Predict Nitrate Exceedances in the Ogallala Aquifer in Texas. Water 2020, 12, 1023. [Google Scholar] [CrossRef] [Green Version]
- Piroonratana, T.; Wongseree, W.; Assawamakin, A.; Paulkhaolarn, N. Chemometrics and Intelligent Laboratory Systems Classi Fi Cation of Haemoglobin Typing Chromatograms by Neural Networks and Decision Trees for Thalassaemia Screening. Chemom. Intell. Lab. Syst. 2009, 99, 101–110. [Google Scholar] [CrossRef]
- Hapfelmeier, A.; Ulm, K. A New Variable Selection Approach Using Random Forests. Comput. Stat. Data Anal. 2013, 60, 50–69. [Google Scholar] [CrossRef]
- Berg, M.; Luzi, S.; Giger, W.; Trang, P.T.K.; Viet, P.H.; Stüben, D. Arsenic Removal from Groundwater by Household Sand Filters: Comparative Field Study, Model Calculations, and Health Benefits. Environ. Sci. Technol. 2006, 40, 5567–5573. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Li, Z.; Chen, B.; Liang, H.; Zhang, X.; Xu, R.; Li, Z.; Dai, H.; Wei, C.; Liu, S. Chemosphere Comparison of Sand-Based Water Fi Lters for Point-of-Use Arsenic Removal in China. Chemosphere 2017, 168, 155–162. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Mitrakas, M.; Zouboulis, A.I. Arsenic Occurrence in Europe: Emphasis in Greece and Description of the Applied Full-Scale Treatment Plants. Desalin. Water Treat. 2015, 54, 2100–2107. [Google Scholar] [CrossRef]
- Litter, M.I.; Alarcón-Herrera, M.T.; Arenas, M.J.; Armienta, M.A.; Avilés, M.; Cáceres, R.E.; Cipriani, H.N.; Cornejo, L.; Dias, L.E.; Cirelli, A.F.; et al. Small-Scale and Household Methods to Remove Arsenic from Water for Drinking Purposes in Latin America. Sci. Total Environ. 2012, 429, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Jain, C.K.; Singh, R.D. Technological Options for the Removal of Arsenic with Special Reference to South. J. Environ. Manag. 2012, 107, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, F. An Overview of Arsenic Removal Technologies in India. Invert. J. Renew. Energy 2017, 7, 5–16. [Google Scholar] [CrossRef]
- Kurz, E.E.C.; Luong, V.T.; Hellriegel, U.; Leidinger, F.; Luu, T.L.; Bundschuh, J.; Hoinkis, J. Iron-Based Subsurface Arsenic Removal (SAR): Results of a Long-Term Pilot-Scale Test in Vietnam. Water Res. 2020, 181, 115929. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, S.; Zhang, S.; Yang, X.; Huang, Z.; Wang, C.; Wei, Q.; Zhang, G.; Xiao, J.; Jiang, F.; et al. Arsenic Pollution and Its Treatment in Yangzonghai Lake in China: In Situ Remediation. Ecotoxicol. Environ. Saf. J. 2015, 122, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Katsoyiannis, I.A.; Zikoudi, A.; Hug, S.J. Arsenic Removal from Groundwaters Containing Iron, Ammonium, Manganese and Phosphate: A Case Study from a Treatment Unit in Northern Greece. Desalination 2008, 224, 330–339. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, S.H.; Lee, W. Investigating Micellar-Enhanced Ultrafiltration (MEUF) of Mercury and Arsenic from Aqueous Solution Using Response Surface Methodology and Gene Expression Programming. Sep. Purif. Technol. 2021, 281, 119880. [Google Scholar] [CrossRef]
- Figoli, A.; Fuoco, I.; Apollaro, C.; Chabane, M.; Mancuso, R.; Gabriele, B.; De Rosa, R.; Vespasiano, G.; Barca, D.; Criscuoli, A. Arsenic-Contaminated Groundwaters Remediation by Nanofiltration. Sep. Purif. Technol. 2020, 238, 116461. [Google Scholar] [CrossRef]
- Chen, A.S.C.; Wang, L.; Sorg, T.J.; Lytle, D.A. Removing Arsenic and Co-Occurring Contaminants from Drinking Water by Full-Scale Ion Exchange and Point-of-Use/Point-of-Entry Reverse Osmosis Systems. Water Res. 2020, 172, 115455. [Google Scholar] [CrossRef] [PubMed]
- Cañas Kurz, E.E.; Hellriegel, U.; Figoli, A.; Gabriele, B.; Bundschuh, J.; Hoinkis, J. Small-Scale Membrane-Based Arsenic Removal for Decentralized Applications–Developing a Conceptual Approach for Future Utilization. Water Res. 2021, 196, 116978. [Google Scholar] [CrossRef]
- Parga, J.R.; Cocke, D.L.; Valenzuela, J.L.; Gomes, J.A.; Kesmez, M.; Irwin, G.; Moreno, H.; Weir, M. Arsenic Removal via Electrocoagulation from Heavy Metal Contaminated Groundwater in La Comarca Lagunera México. J. Hazard. Mater. 2005, 124, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Roy, A.; Mukherjee, R.; Mondal, M.; Karmakar, S.; Chatterjee, S.; Mukherjee, M.; Bhattacharjee, S.; De, S. A Socio-Economic Study along with Impact Assessment for Laterite Based Technology Demonstration for Arsenic Mitigation. Sci. Total Environ. 2017, 583, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Gupta, A.; Biswas, R.K.; Deb, A.K.; Greenleaf, J.E.; Sengupta, A.K. Well-Head Arsenic Removal Units in Remote Villages of Indian Subcontinent: Field Results and Performance Evaluation. Water Res. 2005, 39, 2196–2206. [Google Scholar] [CrossRef]
- Nath, K.J.; Sharma, V.P. Water and Sanitation in the New Millennium; Springer: New Delhi, India, 2017; pp. 1–254. [Google Scholar] [CrossRef]
- Sarkar, S.; Blaney, L.M.; Gupta, A.; Ghosh, D. Use of ArsenXNp, a Hybrid Anion Exchanger, for Arsenic Removal in Remote Villages in the Indian Subcontinent. React. Funct. Polym. 2007, 67, 1599–1611. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing: R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 25 August 2021).
- Othman, F. Reservoir Inflow Forecasting Using Artificial Neural Network. Int. J. Phys. Sci. 2011, 6, 434–440. [Google Scholar] [CrossRef]
- Python Software Foundation. 2020. Available online: https://www.python.org/psf/ (accessed on 20 August 2021).






| Model | Sum of Squares | DOF | Mean Square | F Value | p Value | R2 | Adj R2 |
|---|---|---|---|---|---|---|---|
| Model for CC | |||||||
| Model | |||||||
| Regression | 2,320,470.04 | 1 | 2,320,470.04 | 5830.94 | <0.001 | 0.73 | 0.73 |
| Residual | 864,364.47 | 2172 | 397.96 | ||||
| Total | 3,184,834.52 | 2173 | |||||
| Validation | |||||||
| Regression | 231,362.2 | 1 | 231,362.2 | 503.18 | <0.001 | 0.68 | 0.67 |
| Residual | 110,810.8 | 241 | 459.96 | ||||
| Total | 342,173 | 242 | |||||
| Model for O & M | |||||||
| Model | |||||||
| Regression | 62,809.09 | 1 | 62,809.09 | 136,954 | <0.001 | 0.98 | 0.98 |
| Residual | 996.11 | 2172 | 0.46 | ||||
| Total | 63,805.20 | 2173 | |||||
| Validation | |||||||
| Regression | 6568.20 | 1 | 6568.20 | 15,062.6 | <0.001 | 0.98 | 0.98 |
| Residual | 105.09 | 241 | 0.44 | ||||
| Total | 6673.29 | 242 |
| Model 1 (CC Cost) | Model 2 (OM Cost) | |||
|---|---|---|---|---|
| Error Functions | Model | Validation | Model | Validation |
| Sum of the square of the error | 1,177,617.43 | 159,938.06 | 1155.35 | 122.50 |
| Sum of absolute error | 9598.53 | 1257.79 | 565.83 | 62.97 |
| Mean square error | 541.43 | 658.18 | 0.53 | 0.50 |
| Mean absolute error | 4.41 | 5.18 | 0.26 | 0.26 |
| Average relative error | 110.38 | 130.06 | 2.49 | 2.48 |
| Hybrid fractional error function | 15,844.99 | 18,761.63 | 2.66 | 2.47 |
| Marquardt’s percent standard | 19.97 | 21.77 | 5.63 | 5.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhattacharya, A.; Sahu, S.; Telu, V.; Duttagupta, S.; Sarkar, S.; Bhattacharya, J.; Mukherjee, A.; Ghosal, P.S. Neural Network and Random Forest-Based Analyses of the Performance of Community Drinking Water Arsenic Treatment Plants. Water 2021, 13, 3507. https://doi.org/10.3390/w13243507
Bhattacharya A, Sahu S, Telu V, Duttagupta S, Sarkar S, Bhattacharya J, Mukherjee A, Ghosal PS. Neural Network and Random Forest-Based Analyses of the Performance of Community Drinking Water Arsenic Treatment Plants. Water. 2021; 13(24):3507. https://doi.org/10.3390/w13243507
Chicago/Turabian StyleBhattacharya, Animesh, Saswata Sahu, Venkatesh Telu, Srimanti Duttagupta, Soumyajit Sarkar, Jayanta Bhattacharya, Abhijit Mukherjee, and Partha Sarathi Ghosal. 2021. "Neural Network and Random Forest-Based Analyses of the Performance of Community Drinking Water Arsenic Treatment Plants" Water 13, no. 24: 3507. https://doi.org/10.3390/w13243507