Preparation of a Novel Activated Carbon from Cassava Sludge for the High-Efficiency Adsorption of Hexavalent Chromium in Potable Water: Adsorption Performance and Mechanism Insight
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation Process of ACDCS and Parameters Optimization
2.3. Characterizations of ACDCS
2.4. Adsorption Experiment
3. Results and Discussion
3.1. Optimization of Preparation Parameters of ACDCS
3.2. Effect of ACDCSoptimal Dosage
3.3. Effect of pH
3.4. Adsorption Kinetics
3.4.1. Effect of Initial Cr (VI) Concentration and Adsorption Time
3.4.2. Kinetics Models
3.4.3. Diffusion Mechanism
3.5. Adsorption Equilibrium
3.5.1. Effect of Temperature
3.5.2. Adsorption Isotherm Models
3.6. Energy Changes during the Adsorption Process
3.6.1. Adsorption Thermodynamics
3.6.2. Average Free Energy
3.6.3. Adsorption Activation Energy
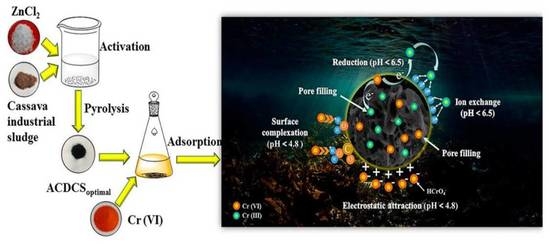
3.7. Adsorption Mechanism
3.7.1. Pore Filling
3.7.2. Electrostatic Attraction
3.7.3. Adsorption-Combined Reduction
3.8. Comparison with Other Adsorbents for Cr (VI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shen, C.; Zhao, Y.; Li, W.; Yang, Y.; Liu, R.; Morgen, D. Global profile of heavy metals and semimetals adsorption using drinking water treatment residual. Chem. Eng. J. 2019, 372, 1019–1027. [Google Scholar] [CrossRef]
- Jia, M.; Peng, L.; Yang, M.; Wei, H.; Zhang, M.; Wang, Y. Carbon dots with dual emission: A versatile sensing platform for rapid assay of Cr (VI). Carbon 2021, 182, 42–50. [Google Scholar] [CrossRef]
- Ding, L.; Jin, X.; Gao, Y.; Ma, J.; Zhang, X. Removal of Cr (VI) from Suddenly Polluted Raw Water Using Miex Resin: Parameters Optimization. Fresenius Environ. Bull. 2019, 28, 5052–5060. [Google Scholar]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Sahinkaya, E.; Altun, M.; Bektas, S.; Komnitsas, K. Bioreduction of Cr(VI) from acidic wastewaters in a sulfidogenic ABR. Miner. Eng. 2012, 32, 38–44. [Google Scholar] [CrossRef]
- Martín-Domínguez, A.; Rivera-Huerta, M.; Pérez-Castrejón, S.; Garrido-Hoyos, S.; Villegas-Mendoza, I.; Gelover-Santiago, S.; Drogui, P.; Buelna, G. Chromium removal from drinking water by redox-assisted coagulation: Chemical versus electrocoagulation. Sep. Purif. Technol. 2018, 200, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Dong, S.; Wang, Y.; Li, J.; Zhang, D.; Zhou, Y.; Tong, Y. Tuning the crosslink structure of cationic hydrogel for enhanced chromium(VI) removal: The covalent and electrostatic co-crosslinked effects and adsorption mechanism. Chem. Eng. J. 2020, 394, 124944. [Google Scholar] [CrossRef]
- Basaran, G.; Kavak, D.; Dizge, N.; Aşçı, Y.; Şölener, M.; Ozbey, B.; Asci, Y. Comparative study of the removal of nickel(II) and chromium(VI) heavy metals from metal plating wastewater by two nanofiltration membranes. Desalin. Water Treat. 2015, 57, 21870–21880. [Google Scholar] [CrossRef]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019, 540, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Li, Q.; Zhang, X.; Liu, L.; Xie, Y.; Guo, L.; Liao, Q.; Yang, Z.; Yang, W. Characteristics, kinetics, thermodynamics and long-term effects of zerovalent iron/pyrite in remediation of Cr(VI)-contaminated soil. Environ. Pollut. 2021, 289, 117830. [Google Scholar] [CrossRef]
- Arroyo, M.; Pérez-Herranz, V.; Montañés, M.; Garcia-Anton, J.; Guiñón, J. Effect of pH and chloride concentration on the removal of hexavalent chromium in a batch electrocoagulation reactor. J. Hazard. Mater. 2009, 169, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Pantazopoulou, E.; Zebiliadou, O.; Noli, F.; Mitrakas, M.; Samaras, P.; Zouboulis, A. Utilization of Phosphogypsum in Tannery Sludge Stabilization and Evaluation of the Radiological Impact. Bull. Environ. Contam. Toxicol. 2014, 94, 352–357. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, Q.; Gao, B.; Gao, Y.; Li, Q.; Wang, Y. Adsorption of hexavalent chromium on Arundo donax Linn activated carbon amine-crosslinked copolymer. Chem. Eng. J. 2012, 217, 240–247. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Liu, M.; Wu, Z.; Yang, L.; Xia, S.; Zhao, J. Cr(VI) removal from water using cobalt-coated bamboo charcoal prepared with microwave heating. Ind. Crops Prod. 2012, 39, 81–88. [Google Scholar] [CrossRef]
- Wu, J.; Yan, X.; Li, L.; Gu, J.; Zhang, T.; Tian, L.; Su, X.; Lin, Z. High-efficiency Adsorption of Cr(VI) and RhB by Hierarchical Porous Carbon Prepared from Coal Gangue. Chemosphere 2021, 275, 130008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, J.; Zhang, W.; Ai, J.; Liu, Y.; Wang, Q.; Wang, D. Preparation of ultrahigh-surface-area sludge biopolymers-based carbon using alkali treatment for organic matters recovery coupled to catalytic pyrolysis. J. Environ. Sci. 2021, 106, 83–96. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, Z.; Xu, S.; Wang, H.; Lu, W. Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresour. Technol. 2015, 190, 388–394. [Google Scholar] [CrossRef]
- Li, M.; Zhou, J.; Bi, Y.-G.; Zhou, S.-Q.; Mo, C.-H. Polypyrrole/sewage sludge carbon as low-cost and high-effective catalyst for enhancing hexavalent chromium reduction and bio-power generation in dual chamber microbial fuel cells. Sep. Purif. Technol. 2020, 256, 117805. [Google Scholar] [CrossRef]
- Mahapatra, U.; Chatterjee, A.; Das, C.; Manna, A.K. Adsorptive removal of hexavalent chromium and methylene blue from simulated solution by activated carbon synthesized from natural rubber industry biosludge. Environ. Technol. Innov. 2021, 22, 101427. [Google Scholar] [CrossRef]
- Cheng, H.; Sun, Y.; Wang, X.; Zou, S.; Ye, G.; Huang, H.; Ye, D. Hierarchical porous carbon fabricated from cellulose-degrading fungus modified rice husks: Ultrahigh surface area and impressive improvement in toluene adsorption. J. Hazard. Mater. 2020, 392, 122298. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Yuan, X.; Li, Y.; Han, L. Effect of pyrolysis temperature and correlation analysis on the yield and physicochemical properties of crop residue biochar. Bioresour. Technol. 2019, 296, 122318. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, Y.; Chen, M.; Ma, L.; Yang, B.; Li, L.; Liu, Q. Optimized preparation of activated carbon from coconut shell and municipal sludge. Mater. Chem. Phys. 2019, 241, 122327. [Google Scholar] [CrossRef]
- Li, X.J.; Hao, J.Y. Orthogonal test design for optimization of synthesis of super early strength anchoring material. Constr. Build. Mater. 2018, 181, 42–48. [Google Scholar] [CrossRef]
- Li, W.-H.; Yue, Q.-Y.; Gao, B.-Y.; Wang, X.-J.; Qi, Y.-F.; Zhao, Y.-Q.; Li, Y.-J. Preparation of sludge-based activated carbon made from paper mill sewage sludge by steam activation for dye wastewater treatment. Desalination 2011, 278, 179–185. [Google Scholar] [CrossRef]
- Yang, B.; Liu, Y.; Liang, Q.; Chen, M.; Ma, L.; Li, L.; Liu, Q.; Tu, W.; Lan, D.; Chen, Y. Evaluation of activated carbon synthesized by one-stage and two-stage co-pyrolysis from sludge and coconut shell. Ecotoxicol. Environ. Saf. 2018, 170, 722–731. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, N.; Wu, H.; Wu, F.A. Preparation of Pruning Mulberry Shoot-based Activated Carbon by ZnCl2 Activation. Adv. Mater. Res. 2011, 282–283, 407–411. [Google Scholar] [CrossRef]
- Tu, B.; Wen, R.; Wang, K.; Cheng, Y.; Deng, Y.; Cao, W.; Zhang, K.; Tao, H. Efficient removal of aqueous hexavalent chromium by activated carbon derived from Bermuda grass. J. Colloid Interface Sci. 2019, 560, 649–658. [Google Scholar] [CrossRef]
- Ma, H.; Yang, J.; Gao, X.; Liu, Z.; Liu, X.; Xu, Z. Removal of chromium (VI) from water by porous carbon derived from corn straw: Influencing factors, regeneration and mechanism. J. Hazard. Mater. 2019, 369, 550–560. [Google Scholar] [CrossRef]
- Al-Othman, Z.; Ali, R.; Naushad, M. Hexavalent chromium removal from aqueous medium by activated carbon prepared from peanut shell: Adsorption kinetics, equilibrium and thermodynamic studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar] [CrossRef]
- Liu, H.; Liang, S.; Gao, J.; Ngo, H.H.; Guo, W.; Guo, Z.; Wang, J.; Li, Y. Enhancement of Cr(VI) removal by modifying activated carbon developed from Zizania caduciflora with tartaric acid during phosphoric acid activation. Chem. Eng. J. 2014, 246, 168–174. [Google Scholar] [CrossRef]
- Duranoğlu, D.; Trochimczuk, A.W.; Beker, U. Kinetics and thermodynamics of hexavalent chromium adsorption onto activated carbon derived from acrylonitrile-divinylbenzene copolymer. Chem. Eng. J. 2012, 187, 193–202. [Google Scholar] [CrossRef]
- Huang, D.; Wang, G.; Li, Z.; Kang, F.; Liu, F. Investigation of the removal mechanism of Cr(VI) in groundwater using activated carbon and cast iron combined system. Environ. Sci. Pollut. Res. 2017, 24, 18341–18354. [Google Scholar] [CrossRef]
- Nasrullah, A.; Saad, B.; Bhat, A.; Khan, A.S.; Danish, M.; Isa, M.H.; Naeem, A. Mangosteen peel waste as a sustainable precursor for high surface area mesoporous activated carbon: Characterization and application for methylene blue removal. J. Clean. Prod. 2018, 211, 1190–1200. [Google Scholar] [CrossRef]
- Nasrullah, A.; Bhat, A.; Naeem, A.; Isa, M.H.; Danish, M. High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int. J. Biol. Macromol. 2017, 107, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Huang, Y.; Wang, H.; Huang, L.; Hu, L.; Zhong, H.; He, Z. A novel composite of almandine supported humboldtine nanospheres, in situ synthesized from natural almandine, possesses high removal efficiency of Cr(VI) over a wide pH range. J. Hazard. Mater. 2019, 383, 121199. [Google Scholar] [CrossRef] [PubMed]
- Aghagoli, M.J.; Shemirani, F. Application of molybdenum disulfide nanosheets for adsorption of tetracycline in water samples. Desalin. Water Treat. 2017, 70, 250–260. [Google Scholar] [CrossRef] [Green Version]
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. Chemcatchem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Equilibria and Capacities for Adsorption on Carbon. J. Sanit. Eng. Div. 1964, 90, 79–108. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Zhang, C.; Ren, L. Preparation and evaluation of activated carbon-based iron-containing adsorbents for enhanced Cr(VI) removal: Mechanism study. Chem. Eng. J. 2012, 189-190, 295–302. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Y.; Jin, L.; Wang, Y.; Qin, M. Adsorption of Cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose. J. Hazard. Mater. 2017, 339, 91–99. [Google Scholar] [CrossRef]
- Ding, L.; Wu, C.; Deng, H.; Zhang, X. Adsorptive characteristics of phosphate from aqueous solutions by MIEX resin. J. Colloid Interface Sci. 2012, 376, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Horsfall, M.J.; Spiff, A.I.; Abia, A. Studies on the Influence of Mercaptoacetic Acid (MAA) Modification of Cassava (Manihot sculenta Cranz) Waste Biomass on the Adsorption of Cu2+ and Cd2+ from Aqueous Solution. Bull. Korean Chem. Soc. 2004, 25, 969–976. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Liu, X.; Li, L.; Shi, J.; Guo, W.; Xue, J. Temporal dynamics of antibiotic resistant genes and their association with the bacterial community in a water-sediment mesocosm under selection by 14 antibiotics. Environ. Int. 2020, 137, 105554. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Bender, S.E.; Wang, J.; Economy, J. Removal of chromium Cr(VI) by low-cost chemically activated carbon materials from water. J. Hazard. Mater. 2009, 166, 74–78. [Google Scholar] [CrossRef]
- Gorzin, F.; Abadi, M.B.R. Adsorption of Cr(VI) from aqueous solution by adsorbent prepared from paper mill sludge: Kinetics and thermodynamics studies. Adsorpt. Sci. Technol. 2018, 36, 149–169. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, J.; Zheng, P.; Qiu, R. Atrazine immobilization on sludge derived biochar and the interactive influence of coexisting Pb(II) or Cr(VI) ions. Chemosphere 2015, 134, 438–445. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, C.; Dan, J.; Pu, W.; Yang, J. Utilization of anaerobic granular sludge for chromium (VI) removal from wastewater: Optimization by response surface methodology. Water Sci. Technol. 2017, 76, 1112–1123. [Google Scholar] [CrossRef]
- Liu, L.; Liu, X.; Wang, D.; Lin, H.; Huang, L. Removal and reduction of Cr(VI) in simulated wastewater using magnetic biochar prepared by co-pyrolysis of nano-zero-valent iron and sewage sludge. J. Clean. Prod. 2020, 257, 120562. [Google Scholar] [CrossRef]
- Rai, M.; Shahi, G.; Meena, V.; Meena, R.; Chakraborty, S.; Singh, R.; Rai, B.N. Removal of hexavalent chromium Cr (VI) using activated carbon prepared from mango kernel activated with H3PO4. Resour. Technol. 2016, 2, S63–S70. [Google Scholar] [CrossRef]
- Selvaraj, K.; Manonmani, S.; Pattabhi, S. Removal of hexavalent chromium using distillery sludge. Bioresour. Technol. 2003, 89, 207–211. [Google Scholar] [CrossRef]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U. Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef] [PubMed]



| Models and Parameters | 1 mg/L | 5 mg/L | 10mg/L |
|---|---|---|---|
| qeexp | 0.99 | 4.13 | 5.66 |
| Pseudo-first-order model | |||
| qecal (mg/g) | 0.93 | 3.59 | 5.11 |
| k1 (mg/(g·min)) | 0.3189 | 0.1049 | 0.1609 |
| R2 | 0.4939 | 0.6580 | 0.6855 |
| SSE | 0.02216 | 1.39233 | 1.37441 |
| Pseudo-second-order model | |||
| qecal (mg/g) | 0.97 | 3.95 | 5.49 |
| k2 (mg/(g·min)) | 0.6849 | 0.0384 | 0.0469 |
| R2 | 0.8761 | 0.8901 | 0.9282 |
| SSE | 0.00542 | 0.44748 | 0.29537 |
| Elovich model | |||
| α (mg/(g·min)) | 8072.6012 | 3.9535 | 39.0889 |
| β (g/mg) | 17.084 | 1.7504 | 1.6394 |
| R2 | 0.9844 | 0.9960 | 0.9823 |
| SSE | 6.83 × 10−4 | 0.01627 | 0.1229 |
| Weber-Morris model | |||
| kid1 (mg/(g·min0.5)) | 0.0431 | 0.3424 | 0.5247 |
| C1 (mg/g) | 0.6846 | 1.3215 | 2.2228 |
| R2 | 0.8553 | 0.9841 | 0.9322 |
| kid2 (mg/(g·min0.5)) | 0.0170 | 0.1845 | 0.1920 |
| C2 (mg/g) | 0.8042 | 2.0196 | 3.5770 |
| R2 | 0.9225 | 0.9162 | 0.9548 |
| Kid3 (mg/(g·min0.5)) | 0.0076 | 0.1011 | 0.1057 |
| C3 (mg/g) | 0.8871 | 2.7024 | 4.2572 |
| R2 | 0.9963 | 0.9263 | 0.9808 |
| Boyd model | |||
| R2 | 0.9833 | 0.9624 | 0.9809 |
| Models and Parameters | 283 K | 293 K | 303 K |
|---|---|---|---|
| qeexp | 6.76 | 7.07 | 8.01 |
| Langmuir model | |||
| qm (mg/g) | 6.6368 | 7.0219 | 7.4593 |
| KL (L/mg) | 0.9441 | 0.9524 | 1.9104 |
| RL | 0.5143–0.0659 | 0.5121–0.06542 | 0.3436–0.03372 |
| R2 | 0.88 | 0.89 | 0.90 |
| Freundlich model | |||
| KF ((mg/g)/(mg/L)1/n) | 3.0958 | 3.3290 | 4.3829 |
| 1/n | 0.3587 | 0.3515 | 0.3044 |
| R2 | 0.98 | 0.99 | 0.99 |
| Temkin model | |||
| AT (L/g) | 27.6142 | 40.1145 | 100.0136 |
| BT (J/mol) | 2.3527 | 2.436 | 2.5191 |
| R2 | 0.91 | 0.90 | 0.92 |
| Dubinin-Radushkevich model | |||
| qmax (mg/g) | 6.210 | 6.628 | 7.678 |
| Kdr (mol2/J2) | 5.0555 × 10−8 | 4.5033 × 10−8 | 2.5372 × 10−8 |
| E (kJ/mol) | 3.14 | 3.33 | 4.44 |
| R2 | 0.87 | 0.87 | 0.93 |
| T (K) | ∆G° (kJ/mol) | ∆S° (J/mol/K) | ∆H° (kJ/mol) |
|---|---|---|---|
| 283 | −10.43 | 36.88 | 10.13 |
| 293 | −10.80 | ||
| 303 | −11.16 | ||
| 313 | −11.53 | ||
| 323 | −11.90 |
| Adsorbents | Adsorption Time | qmax (mg/g) | Dosage (g/L) | pH | Reference |
|---|---|---|---|---|---|
| Paper mill sludge | 3.0 h | 23.18 | 3.5 | 4.0 | [45] |
| Sludge derived biochar | 16.0 h | 16.18 | 1.0 | 5.0 | [46] |
| Anaerobic granular sludge | 2.5 h | 13.19 | 1.5 | 4.5 | [47] |
| Sewage sludge | 24 h | 11.56 | 4.0 | 2.0 | [48] |
| ACDCSoptimal | 3.0 h | 9.84 | 1.0 | 2.0 | This study |
| Peanut shell | 16.0 h | 8.31 | 2.5 | 2.0 | [29] |
| Municipal sludge | 8.0 h | 7.0 | 1.25 | 2.0 | [17] |
| Biochars | 12.0 h | 6.08 | 2.0 | 1.75 | [49] |
| Distillery sludge | 2.0 h | 5.7 | 5.0 | 3.0 | [50] |
| Oak wood | 48.0 h | 5.50 | 10 | 2.0 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Li, Y.; Liu, Y.; Ding, L.; Jin, X.; Lian, H.; Zheng, J. Preparation of a Novel Activated Carbon from Cassava Sludge for the High-Efficiency Adsorption of Hexavalent Chromium in Potable Water: Adsorption Performance and Mechanism Insight. Water 2021, 13, 3602. https://doi.org/10.3390/w13243602
Li L, Li Y, Liu Y, Ding L, Jin X, Lian H, Zheng J. Preparation of a Novel Activated Carbon from Cassava Sludge for the High-Efficiency Adsorption of Hexavalent Chromium in Potable Water: Adsorption Performance and Mechanism Insight. Water. 2021; 13(24):3602. https://doi.org/10.3390/w13243602
Chicago/Turabian StyleLi, Ling, Yan Li, Yiqi Liu, Lei Ding, Xiaopeng Jin, Hongwei Lian, and Jun Zheng. 2021. "Preparation of a Novel Activated Carbon from Cassava Sludge for the High-Efficiency Adsorption of Hexavalent Chromium in Potable Water: Adsorption Performance and Mechanism Insight" Water 13, no. 24: 3602. https://doi.org/10.3390/w13243602