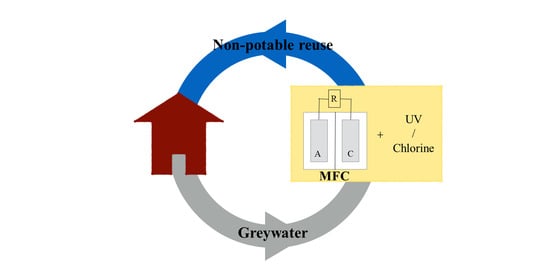
Bioelectrochemical Greywater Treatment for Non-Potable Reuse and Energy Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. MFC Setup
2.2. Greywater Composition
2.3. Monitoring and Analytics
3. Results
4. Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Capodaglio, A.G. Integrated, decentralized wastewater management for resource recovery in rural and peri-urban areas. Resources 2017, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Malila, R.; Lehtoranta, S.; Viskari, E.L. The role of source separation in nutrient recovery—Comparison of alternative wastewater treatment systems. J. Clean. Prod. 2019, 219, 350–358. [Google Scholar] [CrossRef]
- Cecconet, D.; Callegari, A.; Hlavínek, P.; Capodaglio, A.G. Membrane bioreactors for sustainable, fit-for-purpose greywater treatment: A critical review. Clean Technol. Environ. Policy 2019, 21, 745–762. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.G.; Thompson, R.L.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.A.; Ciais, P.; Jackson, R.B.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Sabba, F.; Terada, A.; Wells, G.; Smets, B.F.; Nerenberg, R. Nitrous oxide emissions from biofilm processes for wastewater treatment. Appl. Microbiol. Biotechnol. 2018, 102, 9815–9829. [Google Scholar] [CrossRef] [Green Version]
- Sabba, F.; Picioreanu, C.; Boltz, J.P.; Nerenberg, R. Predicting N2O emissions from nitrifying and denitrifying biofilms: A modeling study. Water Sci. Technol. 2017, 75, 530–538. [Google Scholar] [CrossRef]
- Eriksson, E.; Auffarth, K.; Eilersen, A.M.; Henze, M.; Ledin, A. Household chemicals and personal care products as sources for xenobiotic organic compounds in grey wastewater. Water SA 2003, 29, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Friedler, E.; Hadari, M. Economic feasibility of on-site greywater reuse in multi-storey buildings. Desalination 2006, 190, 221–234. [Google Scholar] [CrossRef]
- Shaikh, I.N.; Ahammed, M.M. Quantity and quality characteristics of greywater: A review. J. Environ. Manag. 2020, 261, 110266. [Google Scholar] [CrossRef]
- Jefferson, B.; Laine, A.; Parsons, S.; Stephenson, T.; Judd, S. Technologies for domestic wastewater recycling. Urban Water 2000, 1, 285–292. [Google Scholar] [CrossRef]
- De Gisi, S.; Casella, P.; Notarnicola, M.; Farina, R. Grey water in buildings: A mini-review of guidelines, technologies and case studies. Civ. Eng. Environ. Syst. 2015, 33, 35–54. [Google Scholar] [CrossRef]
- Atanasova, N.; Dalmau, M.; Comas, J.; Poch, M.; Rodriguez-Roda, I.; Buttiglieri, G. Optimized MBR for greywater reuse systems in hotel facilities. J. Environ. Manag. 2017, 193, 503–511. [Google Scholar] [CrossRef]
- Wu, B. Membrane-based technology in greywater reclamation: A review. Sci. Total Environ. 2019, 656, 184–200. [Google Scholar] [CrossRef]
- Leal, L.H.; Temmink, H.; Zeeman, G.; Cees, C.J. Comparison of three systems for biological greywater treatment. Water 2010, 2, 155–169. [Google Scholar] [CrossRef]
- Arden, S.; Ma, X. Constructed wetlands for greywater recycle and reuse: A review. Sci. Total Environ. 2018, 630, 587–599. [Google Scholar] [CrossRef]
- Bolton, C.R.; Randall, D.G. Development of an integrated wetland microbial fuel cell and sand filtration system for greywater treatment. J. Environ. Chem. Eng. 2019, 7, 103249. [Google Scholar] [CrossRef]
- Cecconet, D.; Omodeo Salè, E.; Callegari, A.; Capodaglio, A.G. Wastewater treatment with a new electrically enhanced biomass concentrator reactor: Trial application and technological perspectives. Environ. Technol. (UK) 2019, 40, 896–902. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Merayo, N.; Prinsen, P.; Luque, R.; Blanco, A.; Zhao, M. A review on greywater reuse: Quality, risks, barriers and global scenarios. Rev. Environ. Sci. Biotechnol. 2019, 18, 77–99. [Google Scholar] [CrossRef]
- Meda, A.; Henkel, J.; Chang, Y.; Cornel, P. Comparison of processes for greywater treatment for urban water reuse: Energy consumption and footprint. In Water-Energy Interactions in Water Reuse; Lazarova, V., Choo, K.-H., Cornel, P., Eds.; IWA Publishing: London, UK, 2012; pp. 203–211. ISBN1 978-1-84339-541-6. ISBN2 978-1-78040-066-2. [Google Scholar]
- Matos, C.; Pereira, S.; Amorim, E.V.; Bentes, I.; Briga-Sá, A. Wastewater and greywater reuse on irrigation in centralized and decentralized systems—An integrated approach on water quality, energy consumption and CO2 emissions. Sci. Total Environ. 2014, 493, 463–471. [Google Scholar] [CrossRef]
- Longo, S.; d’Antoni, B.M.; Bongards, M.; Chaparro, A.; Cronrath, A.; Fatone, F.; Lema, J.M.; Mauricio-Iglesias, M.; Soares, A.; Hospido, A. Monitoring and diagnosis of energy consumption in wastewater treatment plants. A state of the art and proposals for improvement. Appl. Energy 2016, 179, 1251–1268. [Google Scholar] [CrossRef]
- Osset-Álvarez, M.; Rovira-Alsina, L.; Pous, N.; Blasco-Gómez, R.; Colprim, J.; Balaguer, M.D.; Puig, S. Niches for bioelectrochemical systems on the recovery of water, carbon and nitrogen in wastewater treatment plants. Biomass Bioenergy 2019, 130, 105380. [Google Scholar] [CrossRef]
- Sharma, M.; Nandy, A.; Taylor, N.; Venkatesan, S.V.; Ozhukil Kollath, V.; Karan, K.; Thangadurai, V.; Tsesmetzis, N.; Gieg, L.M. Bioelectrochemical remediation of phenanthrene in a microbial fuel cell using an anaerobic consortium enriched from a hydrocarbon-contaminated site. J. Hazard. Mater. 2020, 389, 121845. [Google Scholar] [CrossRef]
- Cecconet, D.; Sabba, F.; Devecseri, M.; Callegari, A.; Capodaglio, A.G. In situ groundwater remediation with bioelectrochemical systems: A critical review and future perspectives. Environ. Int. 2020, 137, 105550. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.A.C.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Jain, A.; He, Z. Cathode-enhanced wastewater treatment in bioelectrochemical systems. NPJ Clean Water 2018, 1, 23. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef]
- Sindhuja, M.; Harinipriya, S.; Bala, A.C.; Ray, A.K. Environmentally available biowastes as substrate in microbial fuel cell for efficient chromium reduction. J. Hazard. Mater. 2018, 355, 197–205. [Google Scholar] [CrossRef]
- Molognoni, D.; Chiarolla, S.; Cecconet, D.; Callegari, A.; Capodaglio, A.G. Industrial wastewater treatment with a bioelectrochemical process: Assessment of depuration efficiency and energy production. Water Sci. Technol. 2018, 77, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Molognoni, D.; Puig, S.; Balaguer, M.D.; Capodaglio, A.G.; Callegari, A.; Colprim, J. Multiparametric control for enhanced biofilm selection in microbial fuel cells. J. Chem. Technol. Biotechnol. 2016, 91, 1720–1727. [Google Scholar] [CrossRef]
- Pinto, R.P.; Srinivasan, B.; Manuel, M.-F.; Tartakovsky, B. A two-population bio-electrochemical model of a microbial fuel cell. Bioresour. Technol. 2010, 101, 5256–5265. [Google Scholar] [CrossRef] [Green Version]
- Capodaglio, A.G.; Callegari, A.; Cecconet, D.; Molognoni, D. Sustainability of decentralized wastewater treatment technologies. Water Pract. Technol. 2017, 12, 463–477. [Google Scholar] [CrossRef]
- Oh, K.S.; Leong, J.Y.C.; Poh, P.E.; Chong, M.N.; Lau, E. Von A review of greywater recycling related issues: Challenges and future prospects in Malaysia. J. Clean. Prod. 2018, 171, 17–29. [Google Scholar] [CrossRef]
- Ghaitidak, D.M.; Yadav, K.D. Characteristics and treatment of greywater—A review. Environ. Sci. Pollut. Res. 2013, 20, 2795–2809. [Google Scholar] [CrossRef]
- Eriksson, E.; Andersen, H.R.; Madsen, T.S.; Ledin, A. Greywater pollution variability and loadings. Ecol. Eng. 2009, 35, 661–669. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Selvam, A.; Cheng, K.Y.; Wong, J.W.C. Influence of ionic conductivity in bioelectricity production from saline domestic sewage sludge in microbial fuel cells. Bioresour. Technol. 2016, 200, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Vologni, V.; Kakarla, R.; Angelidaki, I.; Min, B. Increased power generation from primary sludge by a submersible microbial fuel cell and optimum operational conditions. Bioprocess Biosyst. Eng. 2013, 36, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Coma, M.; Desloover, J.; Boon, N.; Colprim, J.; Balaguer, M.D. Autotrophic denitrification in microbial fuel cells treating low ionic strength waters. Environ. Sci. Technol. 2012, 46, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, B.; Burgess, J.E.; Pichon, A.; Harkness, J.; Judd, S.J. Nutrient addition to enhance biological treatment of greywater. Water Res. 2001, 35, 2702–2710. [Google Scholar] [CrossRef]
- Ge, Z.; Li, J.; Xiao, L.; Tong, Y.; He, Z. Recovery of Electrical Energy in Microbial Fuel Cells. Environ. Sci. Technol. Lett. 2014, 1, 137–141. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.A.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Pant, D.; Logan, B.E. Chronoamperometry and linear sweep voltammetry reveals the adverse impact of high carbonate buffer concentrations on anode performance in microbial fuel cells. J. Power Sources 2020, 476, 228715. [Google Scholar] [CrossRef]
- Sleutels, T.H.J.A.; Hamelers, H.V.M.; Rozendal, R.A.; Buisman, C.J.N. Ion transport resistance in Microbial Electrolysis Cells with anion and cation exchange membranes. Int. J. Hydrogen Energy 2009, 34, 3612–3620. [Google Scholar] [CrossRef]
- Lefebvre, O.; Tan, Z.; Kharkwal, S.; Ng, H.Y. Effect of increasing anodic NaCl concentration on microbial fuel cell performance. Bioresour. Technol. 2012, 112, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.I.; Lee, H.S.; Rittmann, B.E. Carbonate species as OH- carriers for decreasing the pH gradient between cathode and anode in biological fuel cells. Environ. Sci. Technol. 2008, 42, 8773–8777. [Google Scholar] [CrossRef]
- Długołecki, P.; Ogonowski, P.; Metz, S.J.; Saakes, M.; Nijmeijer, K.; Wessling, M. On the resistances of membrane, diffusion boundary layer and double layer in ion exchange membrane transport. J. Memb. Sci. 2010, 349, 369–379. [Google Scholar] [CrossRef]
- Ji, E.; Moon, H.; Piao, J.; Ha, P.T.; An, J.; Kim, D.; Woo, J.J.; Lee, Y.; Moon, S.H.; Rittmann, B.E.; et al. Interface resistances of anion exchange membranes in microbial fuel cells with low ionic strength. Biosens. Bioelectron. 2011, 26, 3266–3271. [Google Scholar] [CrossRef]
- Harnisch, F.; Schröder, U.; Scholz, F. The suitability of monopolar and bipolar ion exchange membranes as separators for biological fuel cells. Environ. Sci. Technol. 2008, 42, 1740–1746. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Logan, B.E. Production of Electricity from Acetate or Butyrate Using a Single-Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef]
- Srikanth, S.; Venkata Mohan, S. Change in electrogenic activity of the microbial fuel cell (MFC) with the function of biocathode microenvironment as terminal electron accepting condition: Influence on overpotentials and bio-electro kinetics. Bioresour. Technol. 2012, 119, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ter Heijne, A.; Strik, D.P.B.T.B.; Hamelers, H.V.M.; Buisman, C.J.N. Cathode potential and mass transfer determine performance of oxygen reducing biocathodes in microbial fuel cells. Environ. Sci. Technol. 2010, 44, 7151–7156. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Ge, Z.; Kelly, P.; Zhang, F.; He, Z. Evaluation of normalized energy recovery (NER) in microbial fuel cells affected by reactor dimensions and substrates. Bioresour. Technol. 2014, 157, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Yoonus, H.; Al-Ghamdi, S.G. Environmental performance of building integrated grey water reuse systems based on Life-Cycle Assessment: A systematic and bibliographic analysis. Sci. Total Environ. 2020, 712, 136535. [Google Scholar] [CrossRef] [PubMed]
- Oteng-Peprah, M.; Acheampong, M.A.; deVries, N.K. Greywater Characteristics, Treatment Systems, Reuse Strategies and User Perception—A Review. Water. Air. Soil Pollut. 2018, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasieva, O.; Sorokin, A.; Szydlowski, L.; Goryanin, I. Do Microbial Fuel Cells have Antipathogenic Properties? J. Comput. Sci. Syst. Biol. 2019, 12, 57–70. [Google Scholar] [CrossRef]
- USEPA 2012 Guidelines for Water Reuse; United States Environmental Protection Agency: Washington, DC, USA, 2012.
- Health Canada. Canadian Guidelines for Domestic Reclaimed Water for Use in Toilet and Urinal Flushing; Health Canada: Ottawa, ON, Canada, 2010. [Google Scholar]
- Li, F.; Wichmann, K.; Otterpohl, R. Evaluation of appropriate technologies for grey water treatments and reuses. Water Sci. Technol. 2009, 59, 249–260. [Google Scholar] [CrossRef]
- Zhu, Z.; Dou, J. Current status of reclaimed water in China: An overview. J. Water Reuse Desalin. 2018, 8, 293–307. [Google Scholar] [CrossRef] [Green Version]
- Maeda, M.; Nakada, K.; Kawamoto, K.; Ikeda, M. Area-wide use of reclaimed water in Tokyo, Japan. Water Sci. Technol. 1996, 33, 51–57. [Google Scholar] [CrossRef]
- Jong, J.; Lee, J.; Kim, J.; Hyun, K.; Hwang, T.; Park, J.; Choung, Y. The study of pathogenic microbial communities in graywater using membrane bioreactor. Desalination 2010, 250, 568–572. [Google Scholar] [CrossRef]
- Nolde, E. Greywater reuse systems for toilet flushing in multi-storey buildings. Urban Water 1999, 1, 275–284. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Markakis, N.; Petousi, I.; Manios, T. Single house on-site grey water treatment using a submerged membrane bioreactor for toilet flushing. Sci. Total Environ. 2016, 551–552, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Pidou, M.; Memon, F.A.; Stephenson, T.; Jefferson, B.; Jeffrey, P. Greywater recycling: Treatment options and applications. Proc. Inst. Civ. Eng. Eng. Sustain. 2007, 160, 119–131. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization; United Nations Environment Program. WHO Guidelines for the Safety Use of Wastewater, Excreta and Greywater; World Health Organization: Geneva (CH), Switzerland, 2006. [Google Scholar]
- Ieropoulos, I.A.; Stinchcombe, A.; Gajda, I.; Forbes, S.; Merino-Jimenez, I.; Pasternak, G.; Sanchez-Herranz, D.; Greenman, J. Pee power urinal-microbial fuel cell technology field trials in the context of sanitation. Environ. Sci. Water Res. Technol. 2016, 2, 336–343. [Google Scholar] [CrossRef] [Green Version]
- Batlle-Vilanova, P.; Rovira-Alsina, L.; Puig, S.; Balaguer, M.D.; Icaran, P.; Monsalvo, V.M.; Rogalla, F.; Colprim, J. Biogas upgrading, CO2 valorisation and economic revaluation of bioelectrochemical systems through anodic chlorine production in the framework of wastewater treatment plants. Sci. Total Environ. 2019, 690, 352–360. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Pasternak, G.; Greenman, J. Urine disinfection and in situ pathogen killing using a Microbial Fuel Cell cascade system. PLoS ONE 2017, 12, e176475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ieropoulos, I.; Obata, O.; Pasternak, G.; Greenman, J. Fate of three bioluminescent pathogenic bacteria fed through a cascade of urine microbial fuel cells. J. Ind. Microbiol. Biotechnol. 2019, 46, 587–599. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Removal of Hepatitis B virus surface HBsAg and core HBcAg antigens using microbial fuel cells producing electricity from human urine. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Cecconet, D.; Bolognesi, S.; Callegari, A.; Capodaglio, A.G. Controlled sequential biocathodic denitrification for contaminated groundwater bioremediation. Sci. Total Environ. 2019, 651, 3107–3116. [Google Scholar] [CrossRef]
- Greenman, J.; Ieropoulos, I.A. Allometric scaling of microbial fuel cells and stacks: The lifeform case for scale-up. J. Power Sources 2017, 356, 365–370. [Google Scholar] [CrossRef]
- Eriksson, E.; Auffarth, K.; Henze, M.; Ledin, A. Characteristics of grey wastewater. Urban Water 2002, 4, 85–104. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Biological combination processes for efficient removal of pharmaceutically active compounds from wastewater: A review and future perspectives. J. Environ. Chem. Eng. 2017, 5, 3590–3603. [Google Scholar] [CrossRef]
- Yan, W.; Xiao, Y.; Yan, W.; Ding, R.; Wang, S.; Zhao, F. The effect of bioelectrochemical systems on antibiotics removal and antibiotic resistance genes: A review. Chem. Eng. J. 2019, 358, 1421–1437. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kim, K.Y.; Resurreccion, E.P.; Lee, W.H. Surfactant addition to enhance bioavailability of bilge water in single chamber microbial fuel cells (MFCs). J. Hazard. Mater. 2019, 368, 732–738. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Q.L.; Wu, M.S. Improved biodegradation of total organic carbon and polychlorinated biphenyls for electricity generation by sediment microbial fuel cell and surfactant addition. RSC Adv. 2015, 5, 62534–62538. [Google Scholar] [CrossRef]
- Wen, Q.; Kong, F.; Ma, F.; Ren, Y.; Pan, Z. Improved performance of air-cathode microbial fuel cell through additional Tween 80. J. Power Sources 2011, 196, 899–904. [Google Scholar] [CrossRef]
- Ren, L.; Tokash, J.C.; Regan, J.M.; Logan, B.E. Current generation in microbial electrolysis cells with addition of amorphous ferric hydroxide, Tween 80, or DNA. Int. J. Hydrogen Energy 2012, 37, 16943–16950. [Google Scholar] [CrossRef]
- Cserháti, T.; Forgács, E.; Oros, G. Biological activity and environmental impact of anionic surfactants. Environ. Int. 2002, 28, 337–348. [Google Scholar] [CrossRef]
- Sathe, S.M.; Bhowmick, G.D.; Dubey, B.K.; Ghangrekar, M.M. Surfactant removal from wastewater using photo-cathode microbial fuel cell and laterite-based hybrid treatment system. Bioprocess Biosyst. Eng. 2020, 43, 2075–2084. [Google Scholar] [CrossRef]
- Callegari, A.; Capodaglio, A.G. Effects of selected industrial pollutants on urban WWTPs activated sludge population, and possible mitigation strategies. Water Pract. Technol. 2017, 12, 619–637. [Google Scholar] [CrossRef]
- Stein, N.E.; Hamelers, H.V.M.; van Straten, G.; Keesman, K.J. Effect of Toxic Components on Microbial Fuel Cell-Polarization Curves and Estimation of the Type of Toxic Inhibition. Biosensors 2012, 2, 255–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, I.; Khuman, C.; Bhowmick, G.; Ghangrekar, M. Upflow microbial fuel cell for removal of emerging contaminants from greywater with concomitant energy recovery. In Proceedings of the IWA Water and Development Ciongress & Exhibition, Colombo, Sri Lanka, 1–5 December 2019; International Water Association: Colombo, Sri Lanka, 2019. [Google Scholar]






| COD | NO2−-N | NO3−-N | NH4+-N | TN | TP | pH | Conductivity | |
|---|---|---|---|---|---|---|---|---|
| mg L−1 | mg L−1 | mg L−1 | mg L−1 | mg L−1 | mg L−1 | - | μS cm−1 | |
| Greywater, low conductivity | 489 | 0.1 | 0 | 0 | 1.8 | 1.13 | 8.65 | 348 |
| Greywater, high conductivity | 489 | 0.1 | 0 | 0 | 1.8 | 1.13 | 8.65 | 1578 |
| Acetate solution | 500 | 0 | 0 | 5.8 | 5.8 | 309.64 | 8.21 | 2360 |
| Test | NERs kWh kgCODrem−1 | CDmax mA L−1 | PDmax mW L−1 | ηCOD % | Vmax mV |
|---|---|---|---|---|---|
| Greywater, low conductivity | 0.15 | 0.97 | 0.38 | 87% | 346 |
| Greywater, high conductivity | 0.21 | 1.13 | 0.46 | 85% | 409 |
| Control, acetate | 0.34 | 1.27 | 0.60 | 90% | 474 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecconet, D.; Bolognesi, S.; Piacentini, L.; Callegari, A.; Capodaglio, A.G. Bioelectrochemical Greywater Treatment for Non-Potable Reuse and Energy Recovery. Water 2021, 13, 295. https://doi.org/10.3390/w13030295
Cecconet D, Bolognesi S, Piacentini L, Callegari A, Capodaglio AG. Bioelectrochemical Greywater Treatment for Non-Potable Reuse and Energy Recovery. Water. 2021; 13(3):295. https://doi.org/10.3390/w13030295
Chicago/Turabian StyleCecconet, Daniele, Silvia Bolognesi, Luca Piacentini, Arianna Callegari, and Andrea G. Capodaglio. 2021. "Bioelectrochemical Greywater Treatment for Non-Potable Reuse and Energy Recovery" Water 13, no. 3: 295. https://doi.org/10.3390/w13030295