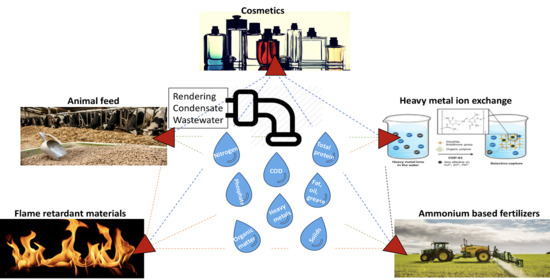
Potential Viable Products Identified from Characterisation of Agricultural Slaughterhouse Rendering Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Materials and Reagents
2.3. Sample Characterisation
2.4. Volatile Fatty Acid Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Raw RCWW
3.1.1. Nutrients
3.1.2. Organic Matter
3.1.3. Volatile Fatty Acids
3.2. Seasonal Variation Analysis
3.3. Identification of Viable By-Products from Rendering Condensate
3.3.1. Ammonium-Based Fertiliser
3.3.2. Animal Feed
3.3.3. Carbon Sources
3.3.4. Heavy Metal Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NH3 | Ammonia |
| NH3OH | Ammonia hydroxide |
| (NH4)2SO4 | Ammonium sulfate |
| ANOVA | Analysis of variance |
| CaHA | Calcium hydroxyapatite |
| COD | Chemical oxygen demand |
| DAF | Dissolved air flotation |
| DO | Dissolved oxygen |
| FOG | Fats, oil & grease |
| FA | Fatty acids |
| GV | Gas chromatography |
| GC-FID | Gas chromatography flame ionisation detection |
| HM | Heavy metals |
| N | Nitrogen |
| NR | Not reported |
| OM | Organic matter |
| P | Phosphorous |
| PTFE | Polytetrafluoroethylene |
| RCWW | Rendering condensate wastewater |
| RWW | Rendering wastewater |
| SHWW | Slaughterhouse wastewater |
| H2SO4 | Sulfuric acid |
| TDS | Total dissolved solids |
| TKN | Total Kjeldahl Nitrogen |
| TN | Total nitrogen |
| TOC | Total organic carbon |
| TP | Total phosphorous |
| TSS | Total suspended solids |
| UF | Ultrafiltration |
| VFA | Volatile fatty acid |
| WW | Wastewater |
References
- Roslan, M.Y.; Debbra, M.; Tan, T.L. Characterisation of wastewater quality from a local ruminant abattoir in Banting, Selangor, Malaysia, Malaysian. J. Vet. Res. 2019, 10, 76–86. [Google Scholar]
- Arvanitoyannis, I.S.; Ladas, D. Meat waste treatment methods and potential uses. Int. J. Food Sci. Technol. 2008, 43, 543–559. [Google Scholar] [CrossRef]
- Metzner, G.; Temper, U. Operation and Optimization of a Full-Scale Fixed-Bed Reactor for Anaerobic Digestion of Animal Rendering Waste Water. Water Sci. Technol. 1990, 22, 373–384. [Google Scholar] [CrossRef]
- Bureau, D. Environmental issues in the rendering industry. Essent. Render. 2003, 1, 245–258. [Google Scholar]
- Bustillo-Lecompte, C.; Mehrvar, E.M. Quiñones-Bolaños, Slaughterhouse wastewater characterization and treatment: An economic and public health necessity of the meat processing industry in Ontario, Canada. Int. Conf. Environ. Pollut. Public Health 2016, 4, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Auterská, P.; Novák, L. Successful solution for high nitrogen content wastewater treatment from rendering plants. Water Sci. Technol. 2006, 54, 23–30. [Google Scholar] [CrossRef]
- Benka-Coker, M.; Ojior, O. Effect of slaughterhouse wastes on the water quality of Ikpoba River, Nigeria. Bioresour. Technol. 1995, 52, 5–12. [Google Scholar] [CrossRef]
- Mkhize, N.T.; Msagati, T.A.; Mamba, B.B.; Momba, M. Determination of volatile fatty acids in wastewater by solvent extraction and gas chromatography. Phys. Chem. Earth 2014, 67–69, 86–92. [Google Scholar] [CrossRef]
- Peu, P.; Béline, F.; Martinez, J. Volatile fatty acids analysis from pig slurry using high-performance liquid chromatography. Int. J. Environ. Anal. Chem. 2004, 84, 1017–1022. [Google Scholar] [CrossRef]
- Hansen, C.L.; West, G.T. Anaerobic digestion of rendering waste in an upflow anaerobic sludge blanket digester. Bioresour. Technol. 1992, 41, 181–185. [Google Scholar] [CrossRef]
- Mulu, A.; Ayenew, T. Characterization of Abattoir Wastewater and Evaluation of the Effectiveness of the Wastewater Treatment Systems in Luna and Kera Abattoirs in Central Ethiopia. Int. J. Sci. Eng. Res. 2015, 6, 1026–1040. Available online: http://www.ijser.org (accessed on 19 March 2020).
- Ula, M.; Trisunaryanti, W.; Falah, I.I.; Kartini, I. Characterization of Gelatines Extracted From Cow Bone for Carbon Synthesis. J. Appl. Chem. 2015, 8, 57–63. Available online: https://pdfs.semanticscholar.org/8b01/3b4cbc9a6ae1cdc3be52ec947898afbc133a.pdf (accessed on 15 October 2019).
- Mainardis, M. Characterization and BMP Tests of Liquid Substrates for High-rate Anaerobic Digestion. Chem. Biochem. Eng. Q. 2018, 31, 509–518. [Google Scholar] [CrossRef]
- Bustillo-Lecompte, C.; Mehrvar, M.; Quiñones-Bolaños, E. Cost-effectiveness analysis of TOC removal from slaughterhouse wastewater using combined anaerobic–aerobic and UV/H2O2 processes. J. Environ. Manag. 2014, 134, 145–152. [Google Scholar] [CrossRef]
- Baker, B.R.; Mohamed, R.; Al-Gheethi, A.; Aziz, H.A. Advanced technologies for poultry slaughterhouse wastewater treatment: A systematic review. J. Dispers. Sci. Technol. 2020, 1–20. [Google Scholar] [CrossRef]
- Edzwald, J.K. Dissolved air flotation and me. Water Res. 2010, 44, 2077–2106. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 1069/2009. Off. J. Eur. Union. 2009, 300, 1–33. [Google Scholar]
- Ware, A.; Power, N. Biogas from cattle slaughterhouse waste: Energy recovery towards an energy self-sufficient industry in Ireland. Renew. Energy 2016, 97, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Wong, D.M.A.L.F. Epidemiology and Control Options of Salmonella in European Pig Herds, Division of Ethology and Health; Royal Veterinary and Agricultural University: Hong Kong, China, 2001. [Google Scholar]
- Lim, S.-J.; Choi, D.W.; Lee, W.G.; Kwon, S.; Chang, H.N. Volatile fatty acids production from food wastes and its application to biological nutrient removal. Bioprocess Biosyst. Eng. 2000, 22, 543–545. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting Nitrogen into Protein—Beyond 6.25 and Jones’ Factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- García-González, M.; Vanotti, M. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of waste strength and pH. Waste Manag. 2015, 38, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Brennan, B.; Briciu-Burghina, C.; Hickey, S.; Abadie, T.; Awali, S.M.A.M.; Delauré, Y.M.C.; Durkan, J.; Holland, L.M.; Quilty, B.; Tajparast, M.; et al. Pilot Scale Study: First Demonstration of Hydrophobic Membranes for the Removal of Ammonia Molecules from Rendering Condensate Wastewater. Int. J. Mol. Sci. 2020, 21, 3914. [Google Scholar] [CrossRef] [PubMed]
- Field, R.A.; Riley, M.L.; Mello, F.C.; Corbridge, J.H.; Kotula, A.W. Bone Composition in Cattle, Pigs, Sheep and Poultry. J. Anim. Sci. 1974, 39, 493–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, C.W.; Porter, J.F.; McKay, G. Removal of Cu(II) and Zn(II) Ions by Sorption onto Bone Char Using Batch Agitation. Langmuir 2002, 18, 650–656. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Waste Water; American Public Health Association: Washington, DC, USA, 1990. [Google Scholar]
- Jiang, C.; Wu, Z.; Zhaoliang, W.; Liu, Q. Technology of protein separation from whey wastewater by two-stage foam separation. Biochem. Eng. J. 2011, 55, 43–48. [Google Scholar] [CrossRef]
- Thayalakumaran, N.; Bhamidimarri, R.; Bickers, P. Characterisation of aerobic bio treatment of meat plant effluent. Water Sci. Technol. 2003, 48, 53–60. [Google Scholar] [CrossRef]
- Sengupta, S.; Nawaz, T.; Beaudry, J. Nitrogen and Phosphorus Recovery from Wastewater. Curr. Pollut. Rep. 2015, 1, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Kartohardjono, S.; Fermi, M.I.; Yuliusman, Y.; Elkardiana, K.; Sangaji, A.P.; Ramadhan, A.M. The Removal of Dissolved Ammonia from Wastewater through a Polypropylene Hollow Fiber Membrane Contactor. Int. J. Technol. 2015, 6, 1146. [Google Scholar] [CrossRef]
- O’Boyle, S.; Trodd, W.; Bradley, C.; Tierney, D.; Wilkes, R.; Longphuirt, S.N.; Smith, J.; Stephens, A.; Barry, J.; Maher, R.P.; et al. Gurrie, Water Quality in Ireland, Wexford. 2019. Available online: www.epa.ie (accessed on 22 September 2020).
- Ruzhitskaya, O.; Gogina, E. Methods for Removing of Phosphates from Wastewater 2 Sources of Phosphates Entering Water Reservoirs. MATEC 2017, 106, 1–7. [Google Scholar]
- Vecino, X.; Reig, M.; Bhushan, B.; Gibert, O.; Valderrama, C.; Cortina, J. Liquid fertilizer production by ammonia recovery from treated ammonia-rich regenerated streams using liquid-liquid membrane contactors. Chem. Eng. J. 2019, 360, 890–899. [Google Scholar] [CrossRef]
- Islam, M.; Shafi, S.; Bandh, S.A.; Shameem, N. Impact of environmental changes and human activities on bacterial diversity of lakes. Freshw. Microbiol. 2019, 105–136. [Google Scholar] [CrossRef]
- Ryan, M.P.; Boyce, A.; Walsh, G. Identification and Evaluation of Phosphorus Recovery Technologies in an Irish Context. 2016. Available online: www.epa.ie (accessed on 19 March 2020).
- Verma, A.; Wei, X.; Kusiak, A. Predicting the total suspended solids in wastewater: A data-mining approach. Eng. Appl. Artif. Intell. 2013, 26, 1366–1372. [Google Scholar] [CrossRef]
- Kurnia, Y.; Agustin, F.; Khalil, G.; Reswati, R. Ferawati Studies on Physical Characteristics, Mineral Composition and Nutritive Value of Bone Meal and Bone Char Produced from Inedible Cow Bones. Pak. J. Nutr. 2017, 16, 426–434. [Google Scholar] [CrossRef] [Green Version]
- Maroneze, M.M.; Barin, J.; De Menezes, C.R.; Queiroz, M.I.; Zepka, L.Q.; Jacob-Lopes, E. Treatment of cattle-slaughterhouse wastewater and the reuse of sludge for biodiesel production by microalgal heterotrophic bioreactors. Sci. Agric. 2014, 71, 521–524. [Google Scholar] [CrossRef] [Green Version]
- Longo, S.; Katsou, E.; Frison, N.; Frison, N.; Renzi, D.; Fatone, F. Recovery of volatile fatty acids from fermentation of sewage sludge in municipal wastewater treatment plants. Bioresour. Technol. 2015, 175, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Harris, P.W.; Lee, S.; McCabe, B.K. Investigating the impact of seasonal temperature variation on biogas production from covered anaerobic lagoons treating slaughterhouse wastewater using lab scale studies. J. Environ. Chem. Eng. 2019, 7. [Google Scholar] [CrossRef]
- Segura, A.A. Ammonium Removal from Wastewater by Liquid-Liquid Membrane Contactors, Universitat Politècnica de Catalunya. 2012. Available online: https://upcommons.upc.edu/handle/2099.1/18480 (accessed on 28 June 2018).
- Lazouski, N.; Chung, M.; Williams, K.; Gala, M.L.; Manthiram, K. Non-aqueous gas diffusion electrodes for rapid ammonia synthesis from nitrogen and water-splitting-derived hydrogen. Nat. Catal. 2020, 3, 463–469. [Google Scholar] [CrossRef]
- Boehler, M.; Heisele, A.; Seyfried, A.; Grömping, M.; Siegrist, H. (NH4)2SO4 recovery from liquid side streams. Environ. Sci. Pollut. Res. 2014, 22, 7295–7305. [Google Scholar] [CrossRef]
- Darestani, M.; Haigh, V.; Couperthwaite, S.J.; Millar, G.J.; Nghiem, L.D. Hollow fibre membrane contactors for ammonia recovery: Current status and future developments. J. Environ. Chem. Eng. 2017, 5, 1349–1359. [Google Scholar] [CrossRef] [Green Version]
- Rezakazemi, M.; Shirazian, S.; Ashrafizadeh, S.N. Simulation of ammonia removal from industrial wastewater streams by means of a hollow-fiber membrane contactor. Desalination 2012, 285, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Lo, Y.M. Recovery of protein from poultry processing wastewater using membrane ultrafiltration. Bioresour. Technol. 2005, 96, 687–698. [Google Scholar] [CrossRef] [PubMed]
- De Lucas, A.; Rodríguez, L.; Villaseñor, J.; Fernández, F. Denitrification potential of industrial wastewaters. Water Res. 2005, 39, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Borja, R.; Banks, C.J.; Wang, Z. Effect of organic loading rate on anaerobic treatment of slaughterhouse wastewater in a fluidised-bed reactor. Bioresour. Technol. 1995, 52, 157–162. [Google Scholar] [CrossRef]
- Vilvert, A.J.; Junior, J.C.S.; Bautitz, I.R.; Zenatti, D.C.; Andrade, M.G.; Hermes, E. Minimization of energy demand in slaughterhouses: Estimated production of biogas generated from the effluent. Renew. Sustain. Energy Rev. 2020, 120, 109613. [Google Scholar] [CrossRef]
- Gunes, B.; Carrié, M.; Benyounis, K.; Stokes, J.; Davis, P.; Connolly, C.; Lawler, J. Optimisation and Modelling of Anaerobic Digestion of Whiskey Distillery/Brewery Wastes after Combined Chemical and Mechanical Pre-Treatment. Processes 2020, 8, 492. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.; Porter, J.; McKay, G. Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char. Water Res. 2001, 35, 605–612. [Google Scholar] [CrossRef]
- Deydier, E.; Guilet, R.; Sharrock, P. Beneficial use of meat and bone meal combustion residue: “An efficient low cost material to remove lead from aqueous effluent”. J. Hazard. Mater. 2003, 101, 55–64. [Google Scholar] [CrossRef]
- Abdel-Halim, S.H.; Shehata, A.M.A.; El-Shahat, M.F. Removal of Zinc and Fluoride Ions from Industrial Waste Water Plants Around Cairo. Bull. Environ. Contam. Toxicol. 2003, 70, 262–267. [Google Scholar] [CrossRef]
- Chojnacka, K. Equilibrium and kinetic modelling of chromium(III) sorption by animal bones. Chemosphere 2005, 59, 315–320. [Google Scholar] [CrossRef]
- Brennan, B.; Lawler, J.; Regan, F. Recovery of viable ammonia–nitrogen products from agricultural slaughterhouse wastewater by membrane contactors: A review. Environ. Sci. Water Res. Technol. 2021. Available online: https://pubs.rsc.org/en/content/articlelanding/2021/ew/d0ew00960a/unauth#!divAbstract (accessed on 17 January 2021). [CrossRef]





| Type of WW | RWW | RWW | SHWW | SHWW | SHWW | Hog WW |
|---|---|---|---|---|---|---|
| pH | 7.5 | NR | 6.5 | 7.3 | 7.2 | 6.9 |
| COD (mg/L) | 9500 | 6000 | 8575 | 11,546 | 109.8 | 8627 |
| TP (mg/L) | 200 | <4 | 112.5 | 202 | 173 | NR |
| TN (mg/L) | 1100 | 430 | 445.5 | 103 | NR | 593 |
| Crude protein (mg/L) | 2187 | 0 | 980 | 375 | 2160 | 1104 |
| FOG (mg/L) | 525 | 110 | 121.5 | 1825 | NR | NR |
| TSS (mg/L) | NR | <6 | 1550 | 3835 | 15.1 | NR |
| HMs (mg/L) | NR | <2 | NR | NR | NR | 369 |
| Nutrients | Organic Matter | Solids | |||
|---|---|---|---|---|---|
| Parameter | Concentration (mg/L) | Parameter | Concentration (mg/L) | Parameter | Concentration (mg/L) |
| TP | 51 ± 1 | DO | 3.1 ± 0.4 | TSS | 336 ± 73 |
| Orthophosphate | 21 ± 0.5 | COD | 10,813 ± 427 | TDS | 4397 ± 405 |
| TN | 2720 ± 82 | TOC | 2513 ± 240 | ||
| TKN | 1630 ± 90 | FOG | 11,363 ± 1942 | ||
| NH3 | 887 ± 21 | pH | 8.34 ± 0.4 | ||
| Crude protein | 10,911 ± 563 | ||||
| Heavy metals and micronutrients (mg/L) | |||||
| P | Copper | Zinc | Lead | Chromium | Iron |
| 2.7 ± 0.1 | 0.01 ± 0 | 0.04 ± 0 | <0.01 | <0.002 | 0.1 ± 0 |
| Potassium | Cobalt | Nickel | Calcium | Magnesium | Sodium |
| 4.1 ± 0 | <0.002 | <0.005 | 10.9 ± 0.1 | 0.8 ± 0.1 | 36.6 ± 1.9 |
| Sulphate | Sulphur | Chloride | Manganese | ||
| 10 ± 0.8 | 68.8 ± 2.5 | 4374 ± 41 | 0.002 ± 0 | ||
| Parameter | Concentration (mg/L) |
|---|---|
| Acetic acid | 1519.7 ± 36.3 |
| Propionic acid | 821.2 ± 17.4 |
| Isobutyric acid | 190.9 ± 15.1 |
| Butyric acid | 738.1 ± 93.4 |
| Isovaleric acid | 233.2 ± 21.8 |
| Valeric acid | 297.4 ± 38.0 |
| Component | Product |
|---|---|
| Nitrogen | Ammonium-based fertiliser; flame-retardant chemicals |
| Volatile fatty acid | Carbon source for denitrification; cosmetics; biogas |
| Protein | Animal feed |
| Calcium hydroxyapatite | Ion-absorber for heavy metals |
| COD | Biogas |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brennan, B.; Gunes, B.; Jacobs, M.R.; Lawler, J.; Regan, F. Potential Viable Products Identified from Characterisation of Agricultural Slaughterhouse Rendering Wastewater. Water 2021, 13, 352. https://doi.org/10.3390/w13030352
Brennan B, Gunes B, Jacobs MR, Lawler J, Regan F. Potential Viable Products Identified from Characterisation of Agricultural Slaughterhouse Rendering Wastewater. Water. 2021; 13(3):352. https://doi.org/10.3390/w13030352
Chicago/Turabian StyleBrennan, Brian, Burcu Gunes, Matthew R. Jacobs, Jenny Lawler, and Fiona Regan. 2021. "Potential Viable Products Identified from Characterisation of Agricultural Slaughterhouse Rendering Wastewater" Water 13, no. 3: 352. https://doi.org/10.3390/w13030352