Diatom Algae-Indicators of Water Quality in the Lower Zarafshan River, Uzbekistan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Area
2.2. Field Sampling
2.3. Laboratory Processing
- Vi, Vj—variable value for adjacent stations i, j;
- l—distance between two adjacent stations (km);
- L—total length of the river between the first and last station.
3. Results
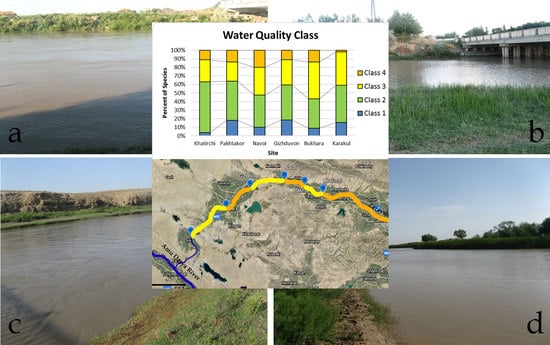
3.1. Physico-Chemical Characteristics of the Lower Part of the Zarafshan River
3.2. Biological Characteristics of the Lower Part of the Zarafshan River
3.3. Bioindicators in the Lower Part of the Zarafshan River
3.4. Indices of Saprobity (SLA) and Ecosystem State (WESI), and River Pollution Index (RPI) in the Lower Part of the Zarafshan River
3.5. Species-Environment Relationships the Lower Part of the Zarafshan River
4. Discussion
4.1. Chemical Variables
4.2. Biological Variables
4.3. Bioindication
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barinova, S. On the Classification of Water Quality from an Ecological Point of View. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 038–045. [Google Scholar] [CrossRef]
- Stevenson, J. Ecological assessments with algae: A review and synthesis. J. Phycol. 2014, 50, 437–461. [Google Scholar] [CrossRef] [PubMed]
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Biggs, B.J.F. Patterns in benthic algae of streams. In Algal Ecology: Freshwater Benthic Ecosystems; Stevenson, R.J., Bothwell, M.L., Lowe, R.L., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 31–56. [Google Scholar]
- Bilous, O. Water Quality and Economy for Sustainable Growth. In Clean Water and Sanitation. Encyclopedia of the UN Sustainable Development Goals; Leal Filho, W., Azul, A.M., Brandli, L., Lange Salvia, A., Wall, T., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Lethier, H. World Heritage thematic study for Central Asia; Priority sites for World Heritage Nomination under Criteria (ix) and (x). IUCN and IUCN ECARO: Gland, Switzerland; Belgrade, Serbia, 2020. [Google Scholar]
- Akhmedzhanov, B.K. Uzbekistan National Report. National Report on the Use and Protection of Water Resources in the Republic of Uzbekistan. Seminar on the Role of Ecosystems as Water Suppliers (Geneva, 13–14 December 2004); Convention on Protection and Use of Transboundary Watercourses and International Lakes: Geneva, Switzerland, 2004; pp. 1–14. (In Russian) [Google Scholar]
- Shultz, V.L.; Mashrapov, R. Hydrography of Central Asia; Teacher: Tashkent, Uzbekistan, 1969; 360p. (In Russian) [Google Scholar]
- Micklin, P.; Aladin, N.V.; Chida, T.; Boroffka, N.; Plotnikov, I.S.; Krivonogov, S.; White, K. The Aral Sea: A Story of Devastation and Partial Recovery of a Large Lake. In Large Asian Lakes in a Changing World. Natural State and Human Impact; Mischke, S., Ed.; Springer: Cham, Switzerland, 2020; Part 4; pp. 109–141. Available online: https://link.springer.com/book/10.1007%2F978-3-030-42254-7 (accessed on 7 January 2021).
- Shultz, V.L. Rivers of Central Asia; LIMIZ: Leningrad, Russia, 1965; 606p. (In Russian) [Google Scholar]
- Uzbek National Encyclopedia; State Scientific Publishing House: Tashkent, Uzbekistan, 2002; Volume 3, p. 704.
- Yearbook: Surface Water Quality in the Territory of UzHYDROMET Activity for 2009–2015; Uzhydromet: Tashkent, Uzbekistan, 2015. (In Russian)
- WWAP (United Nations World Water Assessment Programme)/UN-Water. The United Nations World Water Development Report 2018: Nature-Based Solutions for Water; UNESCO: Paris, France, 2018; 154p. [Google Scholar]
- The Directive 2000/60/EP of the European Parliament and of the Council establishing a framework for community action in the field of water policy. Off. J. Eur. Commun. 2000, L327, 1–73. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32000L0060:en:NOT (accessed on 7 January 2021).
- Dokulil, M.T. Algae as ecological bioindicators. Trace Met. Other Contam. Environ. 2003, 6, 285–327. [Google Scholar]
- Muzafarov, A.M.; Musaev, K.Y. Materials for the knowledge of the flora of algae in water bodies of the upper reaches of the river Zarafshan. In Algae of Reservoirs of Uzbekistan; Publishing House “Fan”: Tashkent, Uzbekistan, 1969; pp. 3–31. (In Russian) [Google Scholar]
- Toshpulatov, Y.S. Taxonomic and floristic analysis of the middle stream algoflora of the Zarafshan river. Bull. Gulistan State Univ. Gulistan 2015, 3, 45–51. (In Uzbek) [Google Scholar]
- Kulmatov, R.; Opp, C.; Groll, M.; Kulmatova, D. Assessment of Water Quality of the Trans-Boundary Zarafshan River in the Territory of Uzbekistan. J. Water Resour. Prot. 2013, 5, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Groll, M.; Opp, C.; Kulmatov, R.; Ikramova, M.; Normatov, I. Water quality, potential conflicts and solutions—An upstream–downstream analysis of the transnational Zarafshan River (Tajikistan, Uzbekistan). Environ. Earth Sci. 2015, 73, 743–763. [Google Scholar] [CrossRef]
- Muzafarov, A.M. Algal Flora of the Central Asian Water Bodies; Uzbekistan Academy of Science Publisher: Tashkent, Uzbekistan, 1965; 570p. (In Russian) [Google Scholar]
- Kiseleva, E.I. Materials for the study of microflora of rice fields in the vicinity of Samarkand. J. Russian Bot. Soc. 1931, 6, 9–11. (In Russian) [Google Scholar]
- Polyansky, V.I. To the flora of algae of Samarkand. Proc. Bot. Inst. Acad. Sci. UssrLeningr. 1950, 5, 5–17. (In Russian) [Google Scholar]
- Ergashev, A.E. On the vegetation of the collector network of the Bukhara region. Uzbek Biol. J. Tashkent 1960, 3, 37–40. (In Russian) [Google Scholar]
- Ergashev, A.E. Algoflora of the main canals of the Fergana Valley. In Algae of Reservoirs of Uzbekistan; Fan: Tashkent, Uzbekistan, 1969; pp. 182–196. (In Russian) [Google Scholar]
- Ergashev, A.E. Characteristic features of the algal flora of the canals of Central Asia. In Canal Hydrobiology, Biological Interference and Their Operation; Naukova Dumka: Kiev, Ukraine, 1972; pp. 42–48. (In Russian) [Google Scholar]
- Ergashev, A.E. Patterns of Development and Distribution of Algal Flora in Artificial Reservoirs of Central Asia; Fan: Tashkent, Uzbekistan, 1976; 360p. (In Russian) [Google Scholar]
- Ergashev, A.E. Saprobic algae found in water bodies of Central Asia and their importance in biological treatment. In Ecological and Physiological Study of Algae and Fungi in Central Asia; Fan: Tashkent, Uzbekistan, 1978; pp. 47–50. (In Russian) [Google Scholar]
- Ergasheva, K.E. Algoflora of the Andijan reservoir and the rivers feeding it. Issues Mod. Algol. 2014, 1. Available online: http://algology.ru/383 (accessed on 29 January 2021). (In Russian).
- Lavoie, I.; Campeau, S.; Darchambeau, F.; Cabana, G.; Dillon, P.J. Are diatoms good integrators of temporal variability in stream water quality? Freshw. Biol. 2007, 53, 827–841. [Google Scholar] [CrossRef]
- Barinova, S.S.; Medvedeva, L.A.; Anissimova, O.V. Diversity of Algal Indicators in Environmental Assessment; Pilies Studio Publisher: Tel Aviv, Israel, 2006; 498p. (In Russian) [Google Scholar]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Prospects; Publishing House of Haifa University: Haifa, Kyiv, Israel, 2019; 367p. (In Russian) [Google Scholar]
- Kholmatov, A.P. State and Prospects of Integrated Water Resources Management in the Zeravshan River Basin. Promoting Integrated Water Resources Management and Transboundary Dialogue in Central Asia, EU-UNDP Project (2009–2012); Ministry of Melioration and Water Resources of the Republic of Tajikistan, United Nations Development Program: Dushanbe, Tajikistan, 2010; 95p. (In Russian) [Google Scholar]
- Barinova, S. Ecological Mapping in Application to Aquatic Ecosystems Bioindication: Problems and Methods. Int. J. Environ. Sci. Nat. Resour. 2017, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Nardini, A.; Schipani, I.; Boz, B. STRARIFLU: A river restoration strategy for Regione Lombardia (I). In CIRF—Italian Centre River Restoration; 2006; Available online: http://www.ors.regione.lombardia.it/OSIEG/AreaAcque/contenuti_informativi/contenuto_informativo_Acqua.shtml?968;www.twole.info (accessed on 7 January 2021).
- Climate-data.org. Available online: https://en.climate-data.org/asia/uzbekistan/bukhara-province/bukhara-614/ (accessed on 7 January 2021).
- Gollerbakh, M.M.; Polyansky, V.I. Keys to Freshwater Algae of the USSR. Issue 1. General Part. Freshwater Algae and Their Study; Soviet Science: Moscow, Russia, 1951; p. 350. (In Russian) [Google Scholar]
- Wasser, S.P. (Ed.) Algae: A Reference Book; Naukova Dumka: Kiev, Ukraine, 1989; 608p. (In Russian) [Google Scholar]
- Kiselev, I.A. Plankton research methods. In Life of Fresh Waters of the USSR.; Publisher AN SSSR: Moscow, Russia; Leningrad, Russia, 1956; Volume 4, pp. 183–200. (In Russian) [Google Scholar]
- Unified Methods of Water Quality Research. Part IV. Methods for Biological and Microbiological Analysis of Waters. Section 2; Resp. for the Issue of Gubachek Z. Council for Mutual Economic Assistance CMEA: Moscow, Russia, 1966; 94p. (In Russian)
- Alekin, O.A.; Semenov, A.D.; Skopintsev, B.A. Manual on the Chemical Analysis of Land Waters; Gidrometeoizdat: Leningrad, Russia, 1973; 268p. (In Russian) [Google Scholar]
- Barinova, S. How to Align and Unify the Cell Counting of Organisms for Bioindication. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 070–072. [Google Scholar] [CrossRef] [Green Version]
- Genkal, S.I.; Chekryzheva, T.A.; Komulainen, S.F. Diatoms of Ponds and Watercourses of Karelia; Scientific World: Moscow, Russia, 2015; 202p. (In Russian) [Google Scholar]
- Key to Freshwater Algae of the USSR; Iss. 4. Diatoms; Soviet Science: Moscow, Russia, 1951; 619p. (In Russian)
- Krammer, K. Diatoms of Europe Vol. 1. Pinnularia; A.R.G. Gantner Verlag. Kommanditgesellschaft: Königstein, Germany, 2000; 703p. [Google Scholar]
- Krammer, K. Diatoms of Europe Vol. 3. Cymbella; A.R.G. Gantner Verlag. Kommanditgesellschaft: Königstein, Germany, 2002; 584p. [Google Scholar]
- Krammer, K. Diatoms of Europe Vol. 4. Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella; A.R.G. Gantner Verlag. Kommanditgesellschaft: Königstein, Germany, 2003; 530p. [Google Scholar]
- Krammer, K. Die cymbelloiden Diatomeen. Teil 1. Allgemeines und Encyonema part. Bibl. Diatomol. 1997, 36, 382. [Google Scholar]
- Krammer, K. Die cymbelloiden Diatomeen. Teil 2. Encyonema part., Encyonopsis und Cymbellopsis. Bibl. Diatomol. 1997, 37, 469. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Achnanthaceae, Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliteratur verzeichnis Teil 1–4. Bacillariophyceae; T. 4. Gustav Fischer Verlag: Stuttgart, Germany; Jena, Germany, 1991; 437p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariaceae, Epithemiaceae, Surirellaceae, Bacillariophyceae; T. 2. Gustav Fischer Verlag: Jena, Germany, 1988; 596p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Centrales, Fragilariaceae, Eunotiaceae, Bacillariophyceae; T. 3. Gustav Fischer Verlag: Stuttgart, Germany; Jena, Germany, 1991; 596p. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Naviculaceae, Bacillariophyceae; T. 1. Gustav Fischer Verlag: Jena, Germany, 1986; 876p. [Google Scholar]
- Kulikovsky, M.S.; Glushchenko, A.M.; Genkal, S.I.; Kuznetsova, I.V. Key to Diatoms of Russia; Filigran: Yaroslavl, Russia, 2016; 804p. (In Russian) [Google Scholar]
- Lange-Bertalot, H. Diatoms of Europe. Navicula Sensu Stricto, 10 Genera Separated from Navicula Sensu Lato, Frustulia; A.R.G. Gantner Verlag K.G.: Ruggel, lLiechtenstein, 2001; Volume 2, 526p. [Google Scholar]
- Lange-Bertalot, H.; Bak, M.; Witkowski, A. Diatoms of Europe. Eunotia and Some Related Genera; A.R.G. Gantner Verlag. Kommanditgesellschaft: Königstein, Germany, 2011; Volume 6, 747p. [Google Scholar]
- Lange-Bertalot, H.; Genkal, S.I. Diatoms from Siberia. I.: Islands in the Arctic Ocean (Yugorsky-SharStrait). Iconogr. Diatomol. 1999, 6, 1–271. [Google Scholar]
- Lange-Bertalot, H.; Genkal, S.I. Diatoms of Siberia. I. Iconogr. Diatomol. 1999, 6, 7–272. [Google Scholar]
- Lange-Bertalot, H.; Metzeltin, D. Indicators of oligotrophy. Iconogr. Diatomol. 1996, 2, 1–390. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication, National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 18 August 2020).
- Sládeček, V. System of water quality from the biological point of view. Arch. Hydrobiol. Beih. Ergeb. Limnol. 1973, 7, 218. [Google Scholar]
- Pantle, R.; Buck, N. Die biologische Überwachung der Gewässer und Darstellung der Ergebnisse. Gas Wasserfach 1955, 96, 604. [Google Scholar]
- Sumita, M. A numerical water quality assessment of rivers in Hokuriku District using epilithic diatom assemblage in river bed as a biological indicator. (II) The values of RPID in surveyed rivers. Diatom. Jpn. J. Diatomol. 1986, 2, 9–18. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power Press: Ithaca, NY, USA, 2002; 500p. [Google Scholar]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, A.J.; Ly, A.; Gronau, Q.F.; Smira, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Krupa, E.; Barinova, S.; Aubakirova, M. Tracking Pollution and its Sources in the Catchment-Lake System of Major Waterbodies in Kazakhstan. Lakes Reserv. Sci. Policy Manag. 2020, 25, 18–30. [Google Scholar] [CrossRef]
- Alimzhanova, K.A. Seasonal changes in algae in the middle part of the Bozsu channel. Uzbek Biol. J. 1997, 2, 37–39. (In Russian) [Google Scholar]
- Alimzhanova, K.A. Regularities in the Distribution of Algae in Water Bodies of the Chirchik River and Their Importance in Determining the Ecological and Sanitary State of Water Bodies; Fan: Tashkent, Uzbekistan, 2007; 264p. (In Russian) [Google Scholar]
- Mamanazarova, K.S. Summer phytoplankton of the lower reaches of the Zarafshan River. In Proceedings of the View of Young Scientists on Topical Problems of Science: Materials of the Republican Scientific and Practical Conference, Tashkent, Uzbekistan, 29 October 2010. [Google Scholar]
- Mamanazarova, K.S.; Alimzhanova, K.A. About the study of algoflora in the Zarafshan river basin. In Proceedings of the Problems of Upbringing a Harmoniously Developed Generation and Ecological Balance: A Collection of Scientific-Practical Seminar Articles, Samarkand, Uzbekistan, 29–30 June 2010; pp. 22–23. (In Russian). [Google Scholar]
- Mamanazarova, K.S. Algoflora of the lower reaches of the Zarafshan River. In Proceedings of the Collection of abstracts 15 of the Pushchino School-Conference of Young Scientists, Pushchino, Russia, 2011; pp. 264–265. (In Russian). [Google Scholar]
- Mamanazarova, K.S. Taxonomic Analysis of Algal Flora of the Lower Reaches of the Zarafshan River; Reports of the Academy of Sciences of the Republic of Uzbekistan: Tashkent, Uzbekistan, 2011; Volume 5, pp. 86–88. (In Russian) [Google Scholar]
- Mamanazarova, K.S.; Alimzhanova, K.A. Diatoms of the lower reaches of the Zarafshan River. Uzbek Biol. J. 2011, 5, 28–31. (In Russian) [Google Scholar]
- Mamanazarova, K.S. Seasonal variation of algae fluorine in the lower reaches of the Zarafshan River. J. Biol. Uzb. 2012, 37–40. (In Uzbek) [Google Scholar]
- Mamanazarova, K.S. Influence of water salinity on the species composition of the algal flora of the lower reaches of the Zarafshan river basin. In Proceedings of the Biosystem: From Theory to Practice: Collection of Theses of the Pushchino School-Conference of Young Scientists, Pushchino, Russia, 24–25 October 2013; p. 85. (In Russian). [Google Scholar]
- Mamanazarova, K.S. Influence of water mineralization on the species composition of algae in the lower reaches of the Zarafshan River. Uzb. Biol. J. 2014, 49–52. (In Russian) [Google Scholar]
- Mamanazarova, K.S. Comparative analysis of algoflora of the upper and lower reaches of the Zarafshan River. In Proceedings of the Problems of Taxonomy and Geography of Aquatic Plants. Materials of the International Conference, Borok, Russia, 2015; pp. 52–53. (In Russian). [Google Scholar]
- Mamanazarova, K.S. Taxonomic analysis of algae-indicators of saprobity in the basin of the lower reaches of the Zarafshan River. In Proceedings of the Ecology of the Native Land: Problems and Ways to Solve Them: Materials of the International Conference, Kirov, Russia, 2016; pp. 156–158. (In Russian). [Google Scholar]
- Niyatbekov, T.; Barinova, S. Diatom Species Richness in Algal Flora of Pamir, Tajikistan. Eur. Sci. J. 2018, 14, 301–323. [Google Scholar] [CrossRef] [Green Version]
- Mamanazarova, K.S.; Gololobova, M.A. First Record of Diatom Species Pleurosira laevis (Ehrenberg) Compère for Uzbekistan and Central Asia. Russ. J. Biol. Invasions 2017, 8, 69–74. (In Russian) [Google Scholar] [CrossRef]
- Alimzhanova, K.A.; Soatov, G.T.; Adylova, M.A. Comparative study of distribution of diatom algae indicators of saprobity in the Kashkadarya River (Uzbekistan). Sci. New Technol. Innov. Kyrg. 2019, 4, 23–27. (In Russian) [Google Scholar]
- Alimjanova, K.A.; Soatov, G.T. Patterns of distribution of algae-indicators of saprobity across the Qashkadaryo River (Republic of Uzbekistan). Algologia 2019, 29, 185–200. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Zarei Darki, B.; Zarei Darki, L.; Akkafi, H.R.; Mirzai, M. Taxonomic Composition of Algae and Its Indicator Role in the Ecosystem of the Zayandehrud River, Iran. Inland Water Biol. 2013, 6, 285–293. [Google Scholar] [CrossRef]
- Krupa, E.G.; Barinova, S.S.; Romanova, S.M. Ecological Mapping in Assessing the Impact of Environmental Factors on the Aquatic Ecosystem of the Arys River Basin, South Kazakhstan. Diversity 2019, 11, 239. [Google Scholar] [CrossRef] [Green Version]
- Barinova, S.S.; Krupa, E.G. Diversity and Ecology of Periphytonic Algae in the Arys River Basin, Kazakhstan. J. Ecol. Nat. Resour. 2017, 1, 1–14. [Google Scholar]
- Barinova, S.; Boboev, M.; Hisoriev, H. Freshwater algal diversity of the South-Tajik Depression in a high mountainous extreme environment. Turk. J. Bot. 2015, 39, 535–546. [Google Scholar] [CrossRef]
- Mamanazarova, K.S. Seasonal development of indicator-system algae of the bottom river basin Zeravshan (Rep. Uzbekistan). Algologia 2014, 24, 67–74. (In Russian) [Google Scholar] [CrossRef] [Green Version]









| Site | No. | Code | North | East | Altitude, m a.s.l. | Distance, km | Number of Species | Sum of Scores | SLA |
|---|---|---|---|---|---|---|---|---|---|
| Khatirchi | 1 | Khat | 40.00.32 | 65.57.10 | 415 | 0 | 38 | 114 | 1.55 |
| Pakhtakor | 2 | Pakh | 40.05.53 | 65.39.26 | 376 | 32.11 | 30 | 82 | 1.47 |
| Navoi | 3 | Navo | 40.09.35 | 65.19.43 | 329 | 34.35 | 52 | 154 | 1.66 |
| Gizhduvon | 4 | Gizh | 40.03.40 | 64.46.25 | 264 | 54.74 | 32 | 88 | 1.45 |
| Bukhara | 5 | Bukh | 39.49.41 | 64.23.17 | 224 | 48.47 | 73 | 197 | 1.53 |
| Karakul | 6 | Kara | 39.30.39 | 63.49.03 | 196 | 63.12 | 84 | 249 | 1.41 |
| Variable | Khatirchi | Average | Pakhtakor | Average | Navoi | Average | Index RPI |
|---|---|---|---|---|---|---|---|
| O2 mg L−1 | 5.2–9.54 | 7.295 | 6.8–8.79 | 7.820 | 5.07–8.44 | 6.983 | 7.477 |
| BOD, mgO L−1 | 0.77–2.7 | 1.638 | 1.36–3.62 | 2.155 | 1.83–3.8 | 2.860 | 2.212 |
| COD, mgO L−1 | 6.75–19.7 | 12.438 | 16.67–35.2 | 26.100 | 19.12–40.9 | 31.125 | 24.098 |
| N-NH4, mg L−1 | 0.02–0.07 | 0.043 | 0.06–0.43 | 0.210 | 0.10–0.25 | 0.165 | 0.158 |
| N-NO2, mg L−1 | 0.01–0.035 | 0.025 | 0.019–0.129 | 0.053 | 0.063–0.178 | 0.120 | 0.064 |
| N-NO3, mg L−1 | 1.09–5.57 | 2.688 | 1.84–3.47 | 2.463 | 2.33–8.79 | 4.830 | 3.129 |
| Fe, mg L−1 | 0.01–0.07 | 0.030 | 0.03–0.14 | 0.073 | 0.04–0.18 | 0.090 | 0.067 |
| Cu, mcg L−1 | 2.6–8.9 | 5.250 | 3.7–8.0 | 5.400 | 4.0–8.2 | 6.100 | 5.545 |
| Zn, mcg L−1 | 2.5–9.7 | 5.650 | 3.1–5.3 | 3.700 | 3.2–5.5 | 4.275 | 4.320 |
| Phenols, mg L−1 | 0.001–0.009 | 0.005 | 0.001–0.003 | 0.001 | 0.001–0.005 | 0.002 | 0.002 |
| Oil, mg L−1 | 0–0.05 | 0.020 | 0–0.01 | 0.005 | 0–0.09 | 0.030 | 0.015 |
| Detergents, mg L−1 | 0–0.05 | 0.015 | 0–0.07 | 0.020 | 0–0.02 | 0.005 | 0.015 |
| TSS, mg L−1 | 198.8–1118 | 591 | 396.9–1250 | 1434 | 200.6–2316 | 1028.1 | 1125.458 |
| DDT, mg L−1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.000 |
| Alpha-HCCG, mcg L−1 | 0 | 0 | 0.001–0.012 | 0.005 | 0.003–0.004 | 0.002 | 0.003 |
| Gamma-HCCG, mcg L−1 | 0 | 0 | 0 | 0 | 0 | 0 | 0.000 |
| Cr VI, mcg L−1 | 0.3–1.5 | 0.825 | 0.6–1.8 | 1.200 | 0.8–1.8 | 1.275 | 1.129 |
| F, mg L−1 | 0.52–1.0 | 0.693 | 0.53–0.96 | 0.780 | 0.63–1.02 | 0.865 | 0.781 |
| As, mcg L−1 | 0–11.0 | 3.125 | 0 | 0 | 0 | 0 | 0.755 |
| TDS, mg L−1 | 537.7–878.1 | 728.8 | 1245.4–1989.5 | 1591.9 | 1330.7–2014.5 | 1710.8 | 1414.124 |
| Indicator Group | Khatirchi | Pakhtakor | Navoi | Gizhduvon | Bukhara | Karakul |
|---|---|---|---|---|---|---|
| Habitat | ||||||
| P | 0.0 | 14.8 | 4.4 | 0.0 | 8.8 | 5.7 |
| P-B | 46.7 | 44.4 | 42.2 | 36.7 | 38.6 | 18.6 |
| B | 53.3 | 40.7 | 53.3 | 63.3 | 52.6 | 75.7 |
| Temperature | ||||||
| cool | 10.0 | 33.3 | 10.0 | 10.0 | 27.3 | 14.3 |
| temp | 80.0 | 66.7 | 80.0 | 70.0 | 36.4 | 78.6 |
| eterm | 10.0 | 0.0 | 10.0 | 10.0 | 27.3 | 0.0 |
| warm | 0.0 | 0.0 | 0.0 | 10.0 | 9.1 | 7.1 |
| Oxygen | ||||||
| st | 5.3 | 0.0 | 10.0 | 15.8 | 24.2 | 18.9 |
| st-str | 78.9 | 68.8 | 63.3 | 68.4 | 57.6 | 59.5 |
| str | 15.8 | 12.5 | 23.3 | 15.8 | 18.2 | 18.9 |
| ae | 0.0 | 18.8 | 3.3 | 0.0 | 0.0 | 2.7 |
| pH | ||||||
| acf | 0.0 | 0.0 | 5.1 | 7.4 | 2.1 | 8.5 |
| ind | 50.0 | 40.0 | 35.9 | 51.9 | 45.8 | 45.8 |
| alf | 50.0 | 60.0 | 59.0 | 40.7 | 52.1 | 44.1 |
| alb | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.7 |
| Salinity | ||||||
| hb | 3.3 | 11.1 | 2.3 | 6.7 | 3.5 | 10.4 |
| i | 80.0 | 66.7 | 72.7 | 63.3 | 70.2 | 62.7 |
| hl | 6.7 | 7.4 | 11.4 | 20.0 | 14.0 | 16.4 |
| mh | 10.0 | 14.8 | 13.6 | 10.0 | 12.3 | 10.4 |
| Saprobity Watanabe | ||||||
| sx | 47.4 | 37.5 | 24.1 | 22.7 | 34.3 | 38.2 |
| es | 52.6 | 62.5 | 72.4 | 68.2 | 57.1 | 55.9 |
| sp | 0.0 | 0.0 | 3.4 | 9.1 | 8.6 | 5.9 |
| Water Quality Class with EU color code and species-specific SLA ranges | ||||||
| Class 1 (0.0–0.5) | 3.7 | 18.2 | 10.0 | 18.5 | 9.1 | 15.7 |
| Class 2 (0.5–1.5) | 59.3 | 45.5 | 37.5 | 40.7 | 34.1 | 43.1 |
| Class 3 (1.5–2.5) | 25.9 | 22.7 | 32.5 | 29.6 | 43.2 | 39.2 |
| Class 4 (2.5–3.5) | 11.1 | 13.6 | 20.0 | 11.1 | 13.6 | 2.0 |
| Trophy | ||||||
| ot | 20.83 | 17.65 | 8.57 | 14.29 | 17.95 | 20.51 |
| o-m | 16.67 | 35.29 | 28.57 | 28.57 | 28.21 | 35.90 |
| m | 4.17 | 0.00 | 5.71 | 4.76 | 5.13 | 7.69 |
| me | 29.17 | 17.65 | 25.71 | 28.57 | 28.21 | 15.38 |
| e | 16.67 | 17.65 | 20.00 | 9.52 | 15.38 | 12.82 |
| o-e | 12.50 | 11.76 | 11.43 | 14.29 | 5.13 | 7.69 |
| Trophy main groups | ||||||
| ot | 37.5 | 52.9 | 37.1 | 42.9 | 46.2 | 56.4 |
| me | 33.3 | 17.6 | 31.4 | 33.3 | 33.3 | 23.1 |
| e | 29.2 | 29.4 | 31.4 | 23.8 | 20.5 | 20.5 |
| Nutrition type | ||||||
| ats | 37.5 | 27.3 | 26.9 | 23.5 | 28.6 | 39.1 |
| ate | 56.3 | 54.5 | 57.7 | 58.8 | 61.9 | 52.2 |
| hne | 0.0 | 0.0 | 7.7 | 17.6 | 9.5 | 8.7 |
| hce | 6.3 | 18.2 | 7.7 | 0.0 | 0.0 | 0.0 |
| Taxa | Khatirchi | Pakhtakor | Navoi | Gizhduvon | Bukhara | Karakul | sLA |
|---|---|---|---|---|---|---|---|
| Cymbella cymbiformis C.Agardh | 0 | 0 | 0 | 0 | 5 | 0 | 2.00 |
| Rhoicosphenia abbreviata (C.Agardh) Lange-Bertalot | 0 | 0 | 5 | 3 | 0 | 3 | 1.90 |
| Sellaphora mutata (Krasske) Lange-Bertalot | 0 | 0 | 0 | 0 | 3 | 5 | 1.90 |
| Sellaphora wummensis J.R.Johansen. | 0 | 0 | 0 | 0 | 5 | 0 | 1.90 |
| Caloneis bacillum (Grunow) Cleve | 0 | 0 | 0 | 0 | 0 | 5 | 1.30 |
| Cocconeis placentula var. euglypta (Ehrenberg) Grunow | 1 | 0 | 0 | 0 | 0 | 5 | 1.30 |
| Gomphonema tergestinum (Grunow) Fricke | 0 | 0 | 0 | 0 | 3 | 5 | 1.30 |
| Synedra famelica Kützing | 0 | 0 | 5 | 0 | 0 | 0 | 1.30 |
| Nupela neogracillima Kulikovskiy & Lange-Bertalot | 0 | 0 | 0 | 0 | 5 | 0 | 1.20 |
| Brachysira microcephala (Grunow) Compère | 5 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Lacustriella lacustris (W.Gregory) Lange-Bertalot & Kulikovskiy | 5 | 0 | 0 | 0 | 0 | 0 | 1.00 |
| Pleurosira laevis (Ehrenberg) Compère | 5 | 5 | 0 | 0 | 0 | 0 | 1.00 |
| Crenotia thermalis (Rabenhorst) Wojtal | 0 | 0 | 0 | 0 | 5 | 0 | 0.30 |
| Index RPI | Index WESI | No of Species | Sum of Scores | Index SLA |
|---|---|---|---|---|
| RPI-1-3 sites | 0.45 | 37.62 | 108.34 | 1.54 |
| RPI-1-6 sites | - | 52.83 | 149.52 | 1.51 |
| Taxa | Code | 1 | 2 | 3 | Hab | T | Oxy | pH | Sal | D | Sap | SLA | Tro | Aut-Het |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brachysira microcephala (Grunow) Compère | BraMic | 5 | 0 | 0 | B | - | - | - | - | - | o | 1.0 | o-m | - |
| Lacustriella lacustris (W.Gregory) Lange-Bertalot & Kulikovskiy | LacLac | 5 | 0 | 0 | B | - | - | ind | i | - | o | 1.0 | o-m | ats |
| Navicula rostellata Kützing | NavRos | 5 | 0 | 5 | B | - | - | - | - | - | - | - | e | - |
| Pleurosira laevis (Ehrenberg) Compère | PleLae | 5 | 5 | 0 | B | temp | - | alf | mh | - | o | 1.0 | e | - |
| Rhoicosphenia abbreviata (C.Agardh) Lange-Bertalot | RhoAbb | 0 | 0 | 5 | B | - | st-str | alf | i | es | o-a | 1.9 | me | ate |
| Synedra famelica Kützing | SynFam | 0 | 0 | 5 | P-B | - | str | alf | i | es | o | 1.3 | m | ats |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barinova, S.; Mamanazarova, K. Diatom Algae-Indicators of Water Quality in the Lower Zarafshan River, Uzbekistan. Water 2021, 13, 358. https://doi.org/10.3390/w13030358
Barinova S, Mamanazarova K. Diatom Algae-Indicators of Water Quality in the Lower Zarafshan River, Uzbekistan. Water. 2021; 13(3):358. https://doi.org/10.3390/w13030358
Chicago/Turabian StyleBarinova, Sophia, and Karomat Mamanazarova. 2021. "Diatom Algae-Indicators of Water Quality in the Lower Zarafshan River, Uzbekistan" Water 13, no. 3: 358. https://doi.org/10.3390/w13030358