1. Introduction
French Vertical Flow (VF) treatment wetlands have become a popular technology to treat domestic wastewater for communities under 5000 people equivalent (p.e.) in France [
1]. French VF treatment wetlands consist of an arrangement of filters in two successive stages planted with
Phragmites Australis. The first stage filters receive raw wastewater in intermittent batches over 3.5 days and then rest for 7 days; three filters are sequentially fed to ensure continuous wastewater treatment. These filters are filled with a coarse granular material (2–4 mm gravel) that allows retaining Suspended Solids (SS). In being fed by raw wastewater, they develop a surface deposit layer [
2] after few years in operation. SS are mainly retained by deep bed filtration [
3] in newly commissioned filters, as well as by cake filtration once the deposit layer has developed.
The deposit layer helps establish a homogeneous distribution of the influent over the treatment wetland surface during a given batch. Moreover, it is thought to be the seat of most biological processes that reduce the concentration of pollutants, including the degradation of Organic Matter (OM) and nitrification provided effective oxygenation can be ensured [
2]. Once this layer reaches a thickness of 20 cm, its removal is recommended in order to renew the infiltration capacity of filters and prevent overflow. At the time of their removal, solids from the deposit layer show a high level of mineralization and stability, which makes the layer suitable for agricultural purposes [
4].
Clogging and fouling, resulting from the accumulation of solids in the filters, are two major concerns in treatment wetland performance and operations. The literature however only contains a few works providing a practical definition of clogging, the degree of clogging [
5], and its difference with fouling, especially in the context of French VF treatment wetlands. Pucher and Langergraber [
5] suggest that a filter should be considered clogged if the volume of water received during a batch does not completely infiltrate before the subsequent batch, moreover, the degree of clogging can be defined as a function of the percentage of the filter surface permanently ponded during two consecutive batches. However, this definition only applies to filters permanently fed and proves to be ill-suited for French VF treatment wetlands, in which ponding duration varies during a feeding period with a strong seasonality effect. The following distinction is therefore preferred in the context of French VF treatment wetlands: a filter is considered clogged if the reduction in infiltration rate leads to overflows or degraded treatment. In contrast, a fouled French VF treatment wetland retains a certain amount of solids within both the filtering gravel layer and deposit layer yet without compromising treatment efficiency. This solid accumulation is herein referred to as biosolids. The biosolids contained in the gravel and deposit layers play an important role in wastewater treatment as a seat of the microorganisms and fauna responsible for organic matter degradation. Biosolids are characterized by their high organic matter content, which ranges from 50% to nearly 90% [
6] and forms a porous medium in which its structure and consistency evolve over time due to water content changes, organic matter mineralization, erosion and bioturbation.
The rest period avoids over-accumulation of biosolids, which could transition the treatment wetland to a clogged state. During this period, the water content and volume of biosolids decrease through drainage, evapotranspiration and mineralization. Biosolids drying leads to shrinkage and cracking, which in turn facilitate oxygen diffusion into the filter and aerobic mineralization.
Understanding the underpinnings of solid transport and its biodegradation is necessary in order to optimize the design and operating rules of French VF treatment wetlands, especially in scenarios aimed at reducing the footprint of the technology or predicting its lifespan. Although the duration of the feeding and rest periods in Reference [
4] has been statistically proven to ensure a good treatment performance in French VF treatment wetlands [
1], these rules remain mainly empirical [
7] and could be optimized. In addition, a mechanistic description of the physical and biological processes in French VF treatment wetlands is key to both understanding the dynamics of clogging, and finding solutions in cases where clogging has occurred prematurely. Taken as a whole, this description would improve the reliability and sustainability of the French VF treatment wetland technology.
Clogging is strongly correlated with the physical, chemical, biological and hydraulic processes in French VF treatment wetlands. Consequently, various methodologies to assess clogging in treatment wetlands are cited in the literature and based on the measurement and monitoring of several variables or properties related to these processes, including: (free)-porosity, solid densities, resistance to erosion, Specific Surface Areas (SSA), chemical composition of biosolids, water infiltration rate, in/ex-situ water permeability, water content, water residence time, oxygen consumption rate, enzymatic activity, oxydo-reduction potential, and even DNA sequencing [
8]. However, fouling and clogging are phenomena that basically consist of filling a void volume by biosolids, where both their amount and geometric distribution are critical.
Only three methodologies that assess clogging/fouling in treatment wetlands and rely on a geometric description are reported in the literature, namely: geo-endoscopy [
9], thin sections [
10], and X-ray Tomography [
11]. Geo-endoscopy yields (2D) images 6 mm high and 5 mm wide with a resolution of 10
m·pixel
and capable of capturing the vertical profile of solids along with the porosity filling level in a filter along a borehole. Thin sections are wider (5 cm × 7 cm); hence, their resolution can reach values of 1
m·pixel
, which makes it possible to observe the biosolid microporosity. However, their production requires the use of organic solvents to replace water in the samples, which in turn may alter the biosolid structure, especially in the deposit layer [
12]. X-ray Computed Tomography (X-ray CT) can yield 3D-morphological descriptions of larger volumes of treatment wetland samples without any pretreatment. The resolution offers a compromise between sample size on the one hand and the size and number of active cells in the X-ray sensor on the other. High-level image processing algorithms are required to process the images generated by X-ray CT.
Recent works on the application of X-ray CT to study French VF treatment wetlands have demonstrated the feasibility and spatial representativy of this methodology [
11,
13], which sets a solid basis for use in studying dynamic phenomena, such as the drying that occurs during the rest period. X-ray CT seems to be a suitable methodology to evaluate the structural changes taking place in French VF treatment wetlands during the rest period for several reasons: (1) it is by far the most widespread methodology for deriving good morphological descriptions of porous media [
14], (2) it produces a realistic (3D) morphological description, (3) it serves as a non destructive technique, necessitating no pretreatment procedure to obtain images of samples from French VF treatment wetlands, and (4) it has already been successfully used to study the dynamics of drying in other types of porous media [
15,
16,
17,
18].
Methodologies, like X-ray CT, are used to estimate properties of filtering media (e.g., porosity, effective transfer coefficients, and water permeability) based on a realistic description of its pore structure [
19,
20]. This upscaling approach differs from global approaches for which filtering media properties are often obtained by calibration. The major concern when studying the filtering media at the pore scale is the spatial representativity of the measurements and it remains an openly discussed topic in the scientific community. Different approaches and methodologies have been proposed to assess the Representative Elementary Volume (REV), which is the volume at which the averaged value of a property is no longer dependent on the size of the sample [
21,
22,
23]. A recent study on the assessment of spatial representativity of X-ray CT for the study of VFTW showed that samples 5 cm in diameter are large enough to be representative of the heterogeneous volumetric distribution of all the phases (voids, biosolids, and gravel) as long as no reed is present [
13].
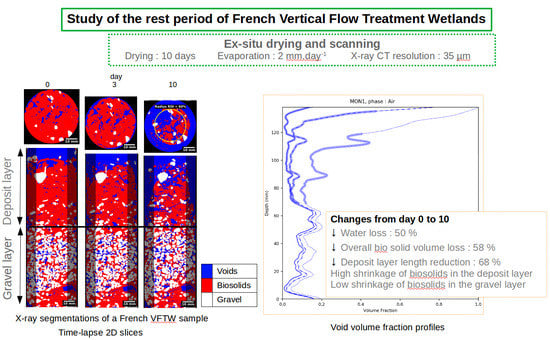
This work carries out an ex situ drying experiment simulating the rest period of a French VF treatment wetland. Three samples from a full scale treatment wetland were left to dry in a closed chamber under controlled conditions of temperature and relative humidity. X-ray CT scans were performed at three different times over a 10-day period. The aim of this experiment was to evaluate how drying affects macropores within the filtering material, as well as their size and connectivity, since this step is believed to be the primary means by which oxygen diffuses into the biosolids and their micropores. We consider here micropores to be voids smaller than 35 µm (scan resolution).
4. Conclusions
This study has demonstrated the applicability of X-ray CT to study structural changes in French VF treatment wetlands at the pore scale during the rest period. An ex situ experiment was performed in a drying chamber in order to simulate a rest period for samples containing the deposit layer and gravel layer of a French VF treatment wetland. An average evaporation rate of 2 was measured; this value is representative of the lowest observations in full-scale treatment wetlands. Under these conditions, drying has induced major changes in the volumetric distribution and morphology of the phases in the samples. At the end of the drying period, the volume of biosolids had been reduced to 58% of its initial value on average. Most of this biosolid volume reduction could be observed in the deposit layer.
This work has confirmed the applicability of X-ray CT to understand the dynamics of structural changes in biosolids and voids within samples from French VF treatment wetlands during an ex situ drying experiment. Clogging is essentially a geometric phenomenon influenced by physical and biological processes in these wetlands; hence, changes in the structure and distribution of biosolids are keys that need to be understood.
The reduction in biosolid volume fraction was 6 times greater in the deposit layer than in the gravel layer; moreover, the deposit layer thickness was reduced to 68% of its initial value. PSD evolved differently during the rest period depending on the given layer. The deposit layer showed an increase in void volume with sizes greater than 4 mm, as a result of radial shrinkage and crack development. When excluding the border effect, a void volume increase with sizes between 0 to 3 mm resulted from drying at the center of the sample. In the gravel layer, the void volume increase was mainly due to an increase in the pores ranging from 0 to 2 mm. The void/biosolid SSA rose from a minimum value of 1.1 to a maximum of 4.2 across the various samples. The SSA and its increase over time were also greater in the deposit layer. The connectivity of the void phase to the atmosphere increased from 19% at the beginning to 91% at the end of the experiment for just one of the samples.
The impact of the rest period on water flow in French VF treatment wetlands has already been discussed qualitatively and quantitatively, but only from a global approach with measurements, like infiltration rates. Nevertheless, the quantification of phenomena at the origin of changes in infiltration rate during the rest period, at the pore scale, has been lacking in the treatment wetland field, yet could be enabled by X-ray CT. Through these measurements, it has been confirmed that nearly all the biosolid structural changes contribute to increasing of the water permeability for the subsequent feeding period, namely: (1) a reduction in the deposit layer thickness, and increases in the (2) void volume, (3) pore size, and (4) connectivity.
By coupling gravimetric measurements with volumetric measurements, the biosolid phase structure can be described beyond the limits of X-ray CT resolution. The air-filled microporosity is another key physical variable to better understand the dynamics of mineralization and clogging, since it controls the oxygen transfer rate within the biosolids and their biological degradation. Based on a hypothesis of the behavior of water/organic matter mixtures, it was possible to estimate an average increase of 0.11 in the air-filled microporosity within the biosolid phase during the drying experiment.
This study demonstrates the suitability of X-ray tomography to measure relevant information of morphological changes in the porous structure of VFTW. These results can notably used to adjust the inlet parameters (porosity, pore size, thickness of the deposit layer) of the numerical models in use to simulate the operation of VFTW. By including the breakthroughs presented in this paper, models may achieve a better mechanistic description of the operation of TW. In order to study the influence of other parameters, like meteorological parameters (air temperature and humidity and wind speed), the methodology introduced in this study could be adapted to perform in situ surveys, notably to study seasonal effects on drying during the rest period. The influence of the changes in the microporous structure on oxygen transport could also be studied to imporve our understanding of the mechanisms that control the rate of aerobic biodegradation of biosolids, which is crucial in delaying the clogging of VFTW.