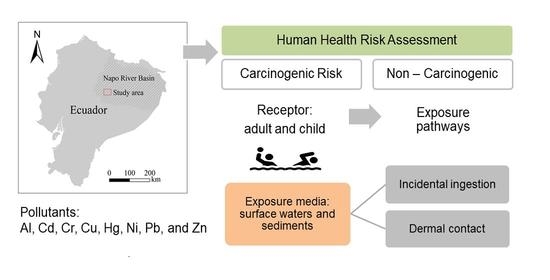
Human Health Risk Assessment for Exposure to Potentially Toxic Elements in Polluted Rivers in the Ecuadorian Amazon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Human Health Risk Assessment
2.2.1. Data Collection and Analyses
2.2.2. Exposure Parameters
2.2.3. Risk Characterization
2.3. Deterministic Approach
2.4. Probabilistic Approach: Monte Carlo Simulation (MCS)
Sensitivity Analysis
| Parameters | Deterministic Approach | Probabilistic Approach | Reference | ||
|---|---|---|---|---|---|
| Point Estimate (RME) | Distribution | Values | |||
| EF | Exposure frequency-adults and children (day/year) | 120 | Triangular | 120 (26–260) | |
| EDa | Exposure duration-adults (year) | 30 | Lognormal | 11.36 ± 13.72 | Israeli et al. [49] |
| EDc | Exposure duration-children (year) | 6 | Uniform | 1–6 | Spence and Walden [50] |
| ET | Exposure time-adults and children (hour/event) | 2.6 | Triangular | 2.6 (0.5–6) | |
| SAa | Skin surface area (swimming)-adults (cm2) | 23,000 | Normal | 18,400 ± 2300 | Anderson et al. [51] |
| SAc | Skin surface area (swimming)-children (cm2) | 7280 | Normal | 6800 ± 600 | Carr [52]; Spence and Walden [50] |
| BWa | Body weight-adults (kg) | 70 | Normal | 72 ± 15.9 | Carr [52] |
| BWc | Body weight-children (kg) | 15 | Normal | 15.6 ± 3.7 | Anderson et al. [51] |
| IRwa | Ingestion rate of water-adults (L/event) | 0.053 | - | 0.053 | USEPA [39] |
| IRwc | Ingestion rate of water-children (L/event) | 0.090 | - | 0.090 | |
| IRsa | Ingestion rate of sediments-adults (mg/event) | 12.5 | - | 12.5 | Goldblum et al. [53] |
| IRsc | Ingestion rate of sediments-children (mg/event) | 50 | - | 50 | |
| ATnc | Averaging time non-carcinogen (day) | 365 × ED | - | 365 × ED | USEPA [21] |
| ATca | Averaging time carcinogen (day) | 365 × 70 | - | 365 × 70 | USEPA [21] |
| AFa | Adherence factor-adults (mg/cm2) | 0.07 | - | 0.07 | USEPA [21] |
| AFc | Adherence factor-children (mg/cm2) | 0.2 | - | 0.2 | USEPA [21] |
| ABS | Dermal absorption factor (unit-less) | 0.001 | - | 0.001 | Wang et al. [9]; USEPA [39] |
| Kp: | Permeability constant (cm/hour) | Al, Cd, Cr, Cu, Hg = 0.001, Pb = 0.0001, Hg, Ni = 0.0002; Zn = 0.0006 | - | Al, Cd, Cr, Cu, Hg = 0.001, Pb = 0.0001, Hg, Ni = 0.0002; Zn = 0.0006 | RAIS [40] |
3. Results
3.1. Deterministic Approach
Point Risk Maps
3.2. Probabilistic Approach: Monte Carlo Simulation (MCS)
3.2.1. Non-Carcinogenic Risk
3.2.2. Carcinogenic Risk
3.2.3. Sensitivity Analysis
4. Discussion
4.1. Potential Impacts of PTEs on Human Health
Deterministic and Probabilistic Quantification
4.2. Environmental Management and Public Policy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pan, L.; Fang, G.; Wang, Y.; Wang, L.; Su, B.; Li, D.; Xiang, B. Potentially Toxic Element Pollution Levels and Risk Assessment of Soils and Sediments in the Upstream River, Miyun Reservoir, China. Int. J. Environ. Res. Public Health 2018, 15, 2364. [Google Scholar] [CrossRef] [Green Version]
- Rehman, I.U.; Ishaq, M.; Ali, L.; Muhammad, S.; Din, I.U.; Yaseen, M.; Ullah, H. Potentially toxic elements’ occurrence and risk assessment through water and soil of Chitral urban environment, Pakistan: A case study. Environ. Geochem. Health 2020, 42, 4355–4368. [Google Scholar] [CrossRef]
- Saha, N.; Rahman, M.S.; Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017, 185, 70–78. [Google Scholar] [CrossRef]
- Guzmán-Martínez, F.; Arranz-González, J.C.; Ortega, M.F.; García-Martínez, M.J.; Rodríguez-Gómez, V. A new ranking scale for assessing leaching potential pollution from abandoned mining wastes based on the Mexican official leaching test. J. Environ. Manag. 2020, 273, 111139. [Google Scholar] [CrossRef] [PubMed]
- WHO. Water Quality Drinking Water, 4th ed.; World Health Organization: Geneva, Switzerland, 2011.
- Castilhos, Z.; Rodrigues-Filho, S.; Cesar, R.; Rodrigues, A.P.; Villas-Bôas, R.; De Jesus, I.; Lima, M.; Faial, K.; Miranda, A.; Brabo, E.; et al. Human exposure and risk assessment associated with mercury contamination in artisanal gold mining areas in the Brazilian Amazon. Environ. Sci. Pollut. Res. 2015, 22, 11255–11264. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-P.; Chen, J.-S.; Chien, Y.-C.; Chen, C.-F. Spatial analysis of the risk to human health from exposure to arsenic contaminated groundwater: A kriging approach. Sci. Total. Environ. 2018, 627, 1048–1057. [Google Scholar] [CrossRef]
- Xu, Z.; Lu, Q.; Xu, X.; Feng, X.; Liang, L.; Liu, L.; Li, C.; Chen, Z.; Qiu, G. Multi-pathway mercury health risk assessment, categorization and prioritization in an abandoned mercury mining area: A pilot study for implementation of the Minamata Convention. Chemosphere 2020, 260, 127582. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, R.; Fan, L.; Chen, T.; Bai, Y.; Yu, Q.; Liu, Y. Assessment of multiple exposure to chemical elements and health risks among residents near Huodehong lead-zinc mining area in Yunnan, Southwest China. Chemosphere 2017, 174, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, X.; Wang, R.; Liu, G. Health risk assessment of potentially harmful elements in subsidence water bodies using a Monte Carlo approach: An example from the Huainan coal mining area, China. Ecotoxicol. Environ. Saf. 2019, 171, 737–745. [Google Scholar] [CrossRef]
- IARC. Monographs on the evaluation of carcinogenic risks to humans. In International Agency for Research on Cancer; World Health Organization: Lyon, France, 1987. [Google Scholar]
- Leikin, J.B.; Paloucek, F.P. Poisoning and Toxicology Handbook, 4th ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2007; ISBN 9780429195648. [Google Scholar] [CrossRef]
- Adimalla, N.; Chen, J.; Qian, H. Spatial characteristics of heavy metal contamination and potential human health risk assessment of urban soils: A case study from an urban region of South India. Ecotoxicol. Environ. Saf. 2020, 194, 110406. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, J.; Wang, Q.; Hong, H.; Zhao, W.; Chen, S.; Yan, C.; Lu, H. Geochemical and probabilistic human health risk of chromium in mangrove sediments: A case study in Fujian, China. Chemosphere 2019, 233, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Gitter, A.; Mena, K.D.; Wagner, K.L.; Boellstorff, D.E.; Borel, K.E.; Gregory, L.F.; Gentry, T.J.; Karthikeyan, R. Human Health Risks Associated with Recreational Waters: Preliminary Approach of Integrating Quantitative Microbial Risk Assessment with Microbial Source Tracking. Water 2020, 12, 327. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zuo, R.; Li, J.; Wu, J.; Zhai, Y.; Teng, Y. The Spatial and Temporal Variability of Groundwater Vulnerability and Human Health Risk in the Limin District, Harbin, China. Water 2018, 10, 686. [Google Scholar] [CrossRef] [Green Version]
- Dooyema, C.A.; Neri, A.; Lo, Y.-C.; Durant, J.; Dargan, P.I.; Swarthout, T.; Biya, O.; Gidado, S.O.; Haladu, S.; Sani-Gwarzo, N.; et al. Outbreak of Fatal Childhood Lead Poisoning Related to Artisanal Gold Mining in Northwestern Nigeria, 2010. Environ. Health Perspect. 2012, 120, 601–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.; Chen, J.; Zhang, J.; Zhang, H.; Qiao, L.; Men, Y. Heavy metals in rice and garden vegetables and their potential health risks to inhabitants in the vicinity of an industrial zone in Jiangsu, China. J. Environ. Sci. 2010, 22, 1792–1799. [Google Scholar] [CrossRef]
- USEPA. Guidelines for Carcinogen Risk Assessment; Environmental Protection Agency: Washington, DC, USA, 2005.
- ACHHRA. Environmental Health Risk Assessment. Guidelines for assessing Human Health Risk from Environmental Hazards; Australian Centre for Human Health Risk Assessment: Melbourne, Australia, 2017. [Google Scholar]
- USEPA. Risk Assessment Guidance for Superfund Volume I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment); Environmental Protection Agency: Washington, DC, USA, 2004.
- Singh, D.D.; Thind, P.S.; Sharma, M.; Sahoo, S.; John, S. Environmentally Sensitive Elements in Groundwater of an Industrial Town in India: Spatial Distribution and Human Health Risk. Water 2019, 11, 2350. [Google Scholar] [CrossRef] [Green Version]
- Castresana, G.P.; Roldán, E.C.; Suastegui, W.A.G.; Perales, J.L.M.; Montalvo, A.C.; Silva, A.H. Evaluation of Health Risks due to Heavy Metals in a Rural Population Exposed to Atoyac River Pollution in Puebla, Mexico. Water 2019, 11, 277. [Google Scholar] [CrossRef] [Green Version]
- Rajasekhar, B.; Nambi, I.M.; Govindarajan, S.K. Human health risk assessment for exposure to BTEXN in an urban aquifer using deterministic and probabilistic methods: A case study of Chennai city, India. Environ. Pollut. 2020, 265, 114814. [Google Scholar] [CrossRef]
- Barrio-Parra, F.; Izquierdo-Díaz, M.; Dominguez-Castillo, A.; Medina, R.; De Miguel, E. Human-health probabilistic risk assessment: The role of exposure factors in an urban garden scenario. Landsc. Urban Plan. 2019, 185, 191–199. [Google Scholar] [CrossRef]
- USEPA. Risk Assessment Guidance for Superfund: Volume III-Part A, Process for Conducting Probabilistic Risk Assessment; Environmental Protection Agency: Washington, DC, USA, 2001.
- Harris, M.J.; Stinson, J.; Landis, W.G. A Bayesian Approach to Integrated Ecological and Human Health Risk Assessment for the South River, Virginia Mercury-Contaminated Site. Risk Anal. 2017, 37, 1341–1357. [Google Scholar] [CrossRef]
- Webb, J.; Mainville, N.; Mergler, D.; Lucotte, M.; Betancourt, O.; Davidson, R.; Cueva, E.; Quizhpe, E. Mercury in Fish-eating Communities of the Andean Amazon, Napo River Valley, Ecuador. EcoHealth 2004, 1, SU59–SU71. [Google Scholar] [CrossRef]
- López-Blanco, C.; Collahuazo, L.; Torres, S.; Chinchay, L.; Ayala, D.; Benítez, P. Mercury Pollution in Soils from the Yacuambi River (Ecuadorian Amazon) as a Result of Gold Placer Mining. Bull. Environ. Contam. Toxicol. 2015, 95, 311–316. [Google Scholar] [CrossRef]
- Mainville, N.; Webb, J.; Lucotte, M.; Davidson, R.; Betancourt, O.; Cueva, E.; Mergler, D. Decrease of soil fertility and release of mercury following deforestation in the Andean Amazon, Napo River Valley, Ecuador. Sci. Total. Environ. 2006, 368, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Jumbo-Flores, D.; González-Merizalde, M.; Bermeo-Flores, S.A.; Alvarez-Figueroa, P.; Mahlknecht, J.; Hernández-Antonio, A. Heavy Metal Enrichment Factors in Fluvial Sediments of an Amazonian Basin Impacted by Gold Mining. Bull. Environ. Contam. Toxicol. 2019, 102, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Requelme, M.R.; Ramos, J.; Angélica, R.; Brabo, E. Assessment of Hg-contamination in soils and stream sediments in the mineral district of Nambija, Ecuadorian Amazon (example of an impacted area affected by artisanal gold mining). Appl. Geochem. 2003, 18, 371–381. [Google Scholar] [CrossRef]
- Vargas, G.C.; Au, W.W.; Izzotti, A. Public health issues from crude-oil production in the Ecuadorian Amazon territories. Sci. Total. Environ. 2020, 719, 134647. [Google Scholar] [CrossRef] [PubMed]
- Maurice, L.; López, F.; Becerra, S.; Jamhoury, H.; Le Menach, K.; Dévier, M.-H.; Budzinski, H.; Prunier, J.; Juteau-Martineau, G.; Ochoa-Herrera, V.; et al. Drinking water quality in areas impacted by oil activities in Ecuador: Associated health risks and social perception of human exposure. Sci. Total. Environ. 2019, 690, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Barraza, F.; Maurice, L.; Uzu, G.; Becerra, S.; López, F.; Ochoa-Herrera, V.; Ruales, J.; Schreck, E. Distribution, contents and health risk assessment of metal(loid)s in small-scale farms in the Ecuadorian Amazon: An insight into impacts of oil activities. Sci. Total. Environ. 2018, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Laraque, A.; Bernal, C.; Bourrel, L.; Darrozes, J.; Christophoul, F.; Armijos, E.; Fraizy, P.; Pombosa, R.; Guyot, J.L. Sediment budget of the Napo River, Amazon basin, Ecuador and Peru. Hydrol. Process. 2009, 23, 3509–3524. [Google Scholar] [CrossRef] [Green Version]
- Capparelli, M.V.; Moulatlet, G.M.; Abessa, D.M.D.S.; Lucas-Solis, O.; Rosero, B.; Galarza, E.; Tuba, D.; Carpintero, N.; Ochoa-Herrera, V.; Cipriani-Avila, I. An integrative approach to identify the impacts of multiple metal contamination sources on the Eastern Andean foothills of the Ecuadorian Amazonia. Sci. Total. Environ. 2020, 709, 136088. [Google Scholar] [CrossRef]
- R Core Team: A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- USEPA. Exposure Factors Handbook: 2011 Edition; Environmental Protection Agency: Washington, DC, USA, 2011.
- Risk Assessment Information System (RAIS), Environmental Protection Agency. Available online: https://rais.ornl.gov/ (accessed on 12 October 2020).
- USEPA. Toxicological Review of Hexavalent Chromium; Environmental Protection Agency: Washington, DC, USA, 1998.
- EFSA. Scientific Opinion on the Risks to Public Health Related to the Presence of Chromium in Food and Drinking Water; European Food Safety Authority: Parma, Italy, 2014. [Google Scholar]
- Alfonso, M.; Ferreira, L.; Durán, R. El Mercurio Como Contaminante Ambiental y Agente Neurotóxico; Universidade de Vigo: Vigo, España, 2010; ISBN 978-84-8158-500-1. [Google Scholar]
- EFSA. Scientific Opinion on the risk for Public Health Related to the Presence of Mercury and Methylmercury in Food; European Food Safety Authority: Parma, Italy, 2012. [Google Scholar]
- Jiménez-Oyola, S.; García-Martínez, M.-J.; Ortega, M.F.; Bolonio, D.; Rodríguez, C.; Esbrí, J.-M.; Llamas, J.F.; Higueras, P.; Oyola, S.J. Multi-pathway human exposure risk assessment using Bayesian modeling at the historically largest mercury mining district. Ecotoxicol. Environ. Saf. 2020, 201, 110833. [Google Scholar] [CrossRef] [PubMed]
- Kaur, L.; Rishi, M.S.; Siddiqui, A.U. Deterministic and probabilistic health risk assessment techniques to evaluate non-carcinogenic human health risk (NHHR) due to fluoride and nitrate in groundwater of Panipat, Haryana, India. Environ. Pollut. 2020, 259, 113711. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Cheng, M.; Zhang, L.; Liu, M.; Yang, X.; Li, X.; Yin, W. The construction dust-induced occupational health risk using Monte-Carlo simulation. J. Clean. Prod. 2018, 184, 598–608. [Google Scholar] [CrossRef]
- Low, K.H.; Zain, S.M.; Abas, M.R.; Salleh, K.M.; Teo, Y.Y. Distribution and health risk assessment of trace metals in freshwater tilapia from three different aquaculture sites in Jelebu Region (Malaysia). Food Chem. 2015, 177, 390–396. [Google Scholar] [CrossRef]
- Israeli, M.; Nelson, C.B. Distribution and Expected Time of Residence for U.S. Households. Risk Anal. 1992, 12, 65–72. [Google Scholar] [CrossRef]
- Spence, L.; Walden, T. RISC4 User’s Manual: Cambridge, UK. 2001. Available online: https://www.groundwatersoftware.com/risc.htm (accessed on 24 February 2021).
- Anderson, E.; Browne, N.; Dulestky, S.; Raming, J.; Warn, T. Development an Statistical Distributions or Ranges of Standard Factos Used in Exposure Assessments, EPA/600/8-85/010; Environmental Protection Agency: Washington, DC, USA, 1985. [Google Scholar]
- Carr, C. American Industrial Health Council: Exposure Factors Sourcebook. Regul. Toxicol. Pharmacol. 1994, 20, 212. [Google Scholar] [CrossRef]
- Goldblum, D.K.; Rak, A.; Ponnapalli, M.D.; Clayton, C.J. The Fort Totten mercury pollution risk assessment: A case history. J. Hazard. Mater. 2006, 136, 406–417. [Google Scholar] [CrossRef]
- Appleton, J.D.; Williams, T.M.; Orbea, H.; Carrasco, M. Fluvial Contamination Associated with Artisanal Gold Mining in the Ponce Enríquez, Portovelo-Zaruma and Nambija Areas, Ecuador. Water Air Soil Pollut. 2001, 131, 19–39. [Google Scholar] [CrossRef]
- González-Merizalde, M.V.; Menezes-Filho, J.A.; Cruz-Erazo, C.T.; Bermeo-Flores, S.A.; Sánchez-Castillo, M.O.; Hernández-Bonilla, D.; Mora, A. Manganese and Mercury Levels in Water, Sediments, and Children Living Near Gold-Mining Areas of the Nangaritza River Basin, Ecuadorian Amazon. Arch. Environ. Contam. Toxicol. 2016, 71, 171–182. [Google Scholar] [CrossRef]
- De Souza, E.S.; Texeira, R.A.; Da Costa, H.S.C.; Oliveira, F.J.; Melo, L.C.A.; Faial, K.D.C.F.; Fernandes, A.R. Assessment of risk to human health from simultaneous exposure to multiple contaminants in an artisanal gold mine in Serra Pelada, Pará, Brazil. Sci. Total. Environ. 2017, 576, 683–695. [Google Scholar] [CrossRef]
- Bonotto, D.M.; Wijesiri, B.; Vergotti, M.; Da Silveira, E.G.; Goonetilleke, A. Assessing mercury pollution in Amazon River tributaries using a Bayesian Network approach. Ecotoxicol. Environ. Saf. 2018, 166, 354–358. [Google Scholar] [CrossRef]
- De Miguel, E.; Clavijo, D.; Ortega, M.F.; Gómez, A. Probabilistic meta-analysis of risk from the exposure to Hg in artisanal gold mining communities in Colombia. Chemosphere 2014, 108, 183–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gochfeld, M. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol. Environ. Saf. 2003, 56, 174–179. [Google Scholar] [CrossRef]







| Surface Water (µg/L) | Sediments (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Min. | p50 | Max. | Std. dev | Min. | p50 | Max. | Std. dev | |
| Al | 0.10 | 0.30 | 303.60 | 83.90 | 2880.80 | 12,365.60 | 269,301.00 | 73,197.12 |
| Cd | 1.70 | 3.00 | 46.00 | 13.60 | 1.40 | 2.70 | 20.10 | 4.84 |
| Cr | 4.90 | 19.40 | 238.30 | 99.82 | 2.70 | 8.90 | 38.80 | 9.63 |
| Cu | 2.80 | 19.20 | 135.30 | 72.23 | 2.40 | 14.35 | 50.80 | 14.78 |
| Hg | 0.50 | 6.70 | 11.20 | 3.97 | 0.10 | 0.10 | 0.40 | 0.13 |
| Ni | 18.10 | 86.85 | 155.60 | 97.23 | 0.90 | 5.60 | 17.20 | 4.53 |
| Pb | 0.70 | 39.30 | 133.10 | 48.14 | 1.00 | 3.15 | 11.00 | 3.10 |
| Zn | 2.40 | 13.05 | 712.00 | 186.17 | 17.80 | 48.30 | 233.70 | 55.06 |
| Surface Waters | Sediments | |||
|---|---|---|---|---|
| Adults | Children | Adults | Children | |
| HI | 1.85 | 4.83 | 1.99 × 102 | 2.66 × 103 |
| TCR | 4.31 × 10−5 | 3.42 × 10−4 | 5.67 × 10−3 | 1.06 × 10−3 |
| Media | PTEs | Fitted Distribution | Distribution Parameters | AD | KS |
|---|---|---|---|---|---|
| Surface water | Al | Cauchy | Location = 0.23, Scale = 0.14 | 3.18 | 0.27 |
| Cd | Cauchy | Location = 2.83, Scale = 0.75 | 1.05 | 0.20 | |
| Cr | Cauchy | Location = 14.40, Scale = 10.85 | 0.70 | 0.27 | |
| Hg | Lognormal | Mean = 1.38, sd = 1.09 | 0.58 | 0.29 | |
| Pb | Logistic | Location = 39.70, Scale = 26.30 | 0.55 | 0.19 | |
| Zn | Lognormal | Mean = 2.71, sd = 1.52 | 0.37 | 0.13 | |
| Sediments | Al | Lognormal | Mean = 9.81, sd = 1.24 | 0.45 | 0.17 |
| Cd | Lognormal | Mean = 1.17, sd = 0.67 | 0.67 | 0.24 | |
| Cr | Logistic | Location = 10.86, Scale = 4.74 | 0.56 | 0.16 | |
| Cu | Lognormal | Mean = 2.44, sd = 0.95 | 0.29 | 0.13 | |
| Hg | Exponential | Rate = 6.25 | 1.11 | 0.46 | |
| Ni | Logistic | Location = 6.56, Scale = 2.37 | 0.82 | 0.15 | |
| Pb | Normal | Mean = 4.06, sd = 2.99 | 0.70 | 0.19 | |
| Zn | Lognormal | Mean = 3.86, sd = 0.68 | 0.26 | 0.11 |
| Surface Water | Sediments | |||
|---|---|---|---|---|
| Adults | Children | Adults | Children | |
| HI | 2.03 × 103 | 3.52 × 103 | 3.69 × 104 | 2.04 × 105 |
| TCR | 2.09 × 10−4 | 7.85 × 10−5 | 1.67 × 10−3 | 8.09 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Oyola, S.; Escobar Segovia, K.; García-Martínez, M.-J.; Ortega, M.; Bolonio, D.; García-Garizabal, I.; Salgado, B. Human Health Risk Assessment for Exposure to Potentially Toxic Elements in Polluted Rivers in the Ecuadorian Amazon. Water 2021, 13, 613. https://doi.org/10.3390/w13050613
Jiménez-Oyola S, Escobar Segovia K, García-Martínez M-J, Ortega M, Bolonio D, García-Garizabal I, Salgado B. Human Health Risk Assessment for Exposure to Potentially Toxic Elements in Polluted Rivers in the Ecuadorian Amazon. Water. 2021; 13(5):613. https://doi.org/10.3390/w13050613
Chicago/Turabian StyleJiménez-Oyola, Samantha, Kenny Escobar Segovia, María-Jesús García-Martínez, Marcelo Ortega, David Bolonio, Iker García-Garizabal, and Bryan Salgado. 2021. "Human Health Risk Assessment for Exposure to Potentially Toxic Elements in Polluted Rivers in the Ecuadorian Amazon" Water 13, no. 5: 613. https://doi.org/10.3390/w13050613