Winter Zooplankton in a Small Arctic Lake: Abundance and Vertical Distribution
Abstract
:1. Introduction
2. Materials and Methods
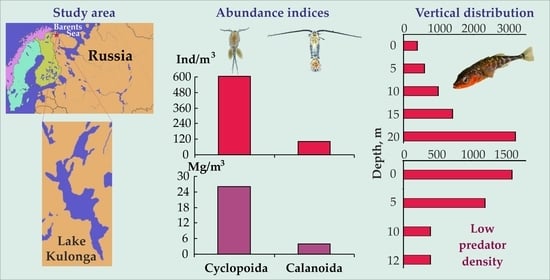
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, R.; Conway, D.V.P.; Hunt, H.G. The role of copepods in the planktonic ecosystems of mixed and stratified waters of the European shelf seas. Hydrobiologia 1994, 292, 521–530. [Google Scholar] [CrossRef]
- Hudson, J.; Taylor, W.; Schindler, D. Planktonic nutrient regeneration and cycling efficiency in temperate lakes. Nature 1999, 400, 659–661. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Macrozooplankton of the Arctic—The Kara Sea in relation to environmental conditions. Estuar. Coast. Shelf Sci. 2017, 88, 38–55. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Mesozooplankton in the Kola Transect (Barents Sea): Autumn and winter structure. J. Sea Res. 2018, 142, 18–22. [Google Scholar] [CrossRef]
- Lampert, W.; Sommer, U. Limnolecology: The Ecology of Lakes and Streams; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Summer macrozooplankton assemblages of Arctic shelf: A latitudinal study. Cont. Shelf Res. 2019, 188, 103967. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Arctic marine mesozooplankton at the beginning of the polar night: A case study for southern and south-western Svalbard waters. Polar Biol. 2020, 43, 71–79. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Summer variability of reproductive pattern in the marine cladoceran Evadne nordmanni in Arctic waters. J. Sea Res. 2020, 166, 101969. [Google Scholar] [CrossRef]
- Beaver, J.R.; Arp, C.D.; Tausz, C.E.; Jones, B.M.; Whitman, M.S.; Renicker, T.R.; Samples, E.E.; Ordosch, D.M.; Scotese, K.C. Potential shifts in zooplankton community structure in response to changing ice regimes and hydrologic connectivity. Arctic Antarctic Alpine Res. 2019, 51, 327–345. [Google Scholar] [CrossRef] [Green Version]
- Rautio, M.; Bayly, I.A.E.; Gibson, J.A.E.; Nyman, M. Zooplankton and zoobenthos in high-latitude water bodies. In Polar Lakes and Rivers: Limnology of Arctic and Antarctic Aquatic Ecosystems; Vincent, W.G., Laybourn-Parry, J., Eds.; Oxford University Press: Oxford, UK, 2000; pp. 231–247. [Google Scholar]
- Fomina, Y.Y.; Syarki, M.T. Modern state of zooplankton and its response to climate change in Petrozavodsk Bay of Lake Onego. Proc. Karelian Res. Centre RAS 2018, 9, 54–64. (In Russian) [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Academic: San Diego, CA, USA, 2001. [Google Scholar]
- Vandysh, O.I. The current state of zooplankton community in Lake Ryashkovskoye (Murmansk oblast). Inland Water Biol. 2010, 3, 135–141. [Google Scholar] [CrossRef]
- Symons, C.C.; Pedruski, M.T.; Arnott, S.E.; Sweetman, J.N. Spatial, environmental, and biotic determinants of zooplankton community composition in subarctic lakes and ponds in Wapusk National Park, Canada. Arctic Antarctic Alpine Res. 2014, 46, 159–190. [Google Scholar] [CrossRef] [Green Version]
- Vandysh, O.I.; Kashulin, N.A.; Cherepanov, A.A. Long-term changes of zooplankton communities in Imandra Lake under multilevel pollution by mining production runoffs. Herald Kola Sci. Centre RAS 2014, 2, 121–129. (In Russian) [Google Scholar]
- Valkova, S.A.; Denisov, D.B.; Terentyev, P.M.; Vandysh, O.I.; Kashulin, N.A. Hydrobiological characteristics of some small lakes in the northern taiga zone (Kola Peninsula). Proc. Karelian Res. Centre. RAS 2015, 4, 79–93. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Hampton, S.E.; Moore, M.V.; Ozersky, T.; Stanley, E.H.; Polashenski, C.M.; Galloway, A.W.E. Heating up a cold subject: Prospects for under-ice plankton research in lakes. J. Plankton Res. 2015, 37, 277–284. [Google Scholar] [CrossRef]
- Berge, J.; Renaud, P.E.; Darnis, G.; Cottier, C.; Last, K.; Gabrielsen, T.M.; Johnsen, G.; Seuthe, L.; Weslawski, J.M.; Leu, E.; et al. In the dark: A review of ecosystem processes during the Arctic polar night. Prog. Oceanogr. 2015, 139, 258–271. [Google Scholar]
- Berge, J.; Cottier, F.; Last, K.S.; Varpe, Ø.; Leu, E.; Soreide, J.; Eiane, K.; Falk-Petersen, S.; Willis, K.; Nygard, H.; et al. Diel vertical migration of Arctic zooplankton during the polar night. Biol. Let. 2009, 5, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Błędzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Filimonova, Z.I. Zooplankton of Mikkelskoe and Kroshnozero lakes and their significance in feeding of fish. Proc. Karel. Branch USSR Acad. Sci. 1956, 2, 89–124. (In Russian) [Google Scholar]
- Filimonova, Z.I. Zooplankton of Kuito lake and their seasonal dynamics. In Conference Abstract of Conference in 1962; Institute of Biology of Karelian Branch of USSR Academy Sciences: Petrozavodsk, Russia, 1963; pp. 148–150. (In Russian) [Google Scholar]
- Carrozzo, D.; Musazzi, S.; Lami, A.; Córdoba, F.E.; González Sagrario, M.Á. Changes in planktivory and herbivory regimes in a shallow south american lake (Lake Blanca Chica, Argentina) over the last 250 years. Water 2020, 12, 597. [Google Scholar] [CrossRef] [Green Version]
- Magnuson, J.J.; Robertson, D.M.; Benson, B.J.; Wynne, R.H.; Livingstone, D.M.; Arai, T.; Assel, R.A.; Barry, R.G.; Card, V.; Kuusisto, E.; et al. Historical trends in lake and river ice cover in the northern hemisphere. Science 2000, 289, 1743–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frolov, A.A. Description of the study area. In Engineering and Environmental Research for the Object Center for Construction of Large-Capacity Marine Structures (CSCMS) in the Village Area Belokamenka. Volume 2 (Lake Kulonga); Ishkulov, D.G., Frolov, A.A., Eds.; MMBI KSC RAS Press: Murmansk Russia, 2016; pp. 6–11. (In Russian) [Google Scholar]
- Einsle, U. Copepoda: Cyclopoida, Genera Cyclops, Megacyclops, Acanthocyclops. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World No.10; SPB Academic Publishing: London, UK, 1996. [Google Scholar]
- Båtnes, A.S.; Miljeteig, C.; Berge, J.; Greenacre, M.; Johnsen, G. Quantifying the light sensitivity of Calanus spp. during the polar night: Potential for orchestrated migrations conducted by ambient light from the sun, moon, or aurora borealis? Polar Biol. 2015, 38, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Chislenko, L.L. Nomogrammes to Determine Weights of Aquatic Organisms Based on the Size and form of Their Bodies (Marine Mesobenthos and Plankton); Nauka Press: Saint Petersburg, Russia, 1968. (In Russian) [Google Scholar]
- McCauley, E. The estimation of the abundance and biomass of zooplankton in samples. In Manual on Methods for the Assessment of Secondary Productivity in Fresh Water; Downing, J.A., Rigler, F.H.A., Eds.; Blackwell: St Louis, MO, USA, 1984; pp. 228–265. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v5: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2001. [Google Scholar]
- Ditton, R.B.; Holland, S.M.; Anderson, D.K. Recreational fishing as tourism. Fisheries 2002, 27, 17–24. [Google Scholar] [CrossRef]
- Syarki, M.T.; Fomina, Y.Y. Current state of zooplankton of Lake Munozero (Republic of Karelia, Russia). Ecosyst. Transform. 2020, 3, 11–18. [Google Scholar] [CrossRef]
- Kulikova, T.P. Zooplankton in Waters of the White Sea Drainage Basin; Karelian Res. Centre, Russian Acad. Sci.: Petrozavodsk, Russia, 2010. (In Russian) [Google Scholar]
- Dvoretsky, V.G.; Dvoretsky, A.G. Distribution of the under-ice mesozooplankton in the Kara Sea (February 2002). Polar Biol. 2009, 32, 1227–1231. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Early winter mesozooplankton of the coastal south-eastern Barents Sea. Estuar. Coast. Shelf Sci. 2015, 152, 116–123. [Google Scholar] [CrossRef]
- Berge, J.; Cottier, F.; Varpe, Ø.; Renaud, P.E.; Falk-Petersen, S.; Kwasniewski, S.; Griffiths, C.; Søreide, J.E.; Johnsen, G.; Aubert, A.; et al. Arctic complexity: A case study on diel vertical migration of zooplankton. J. Plankton Res. 2014, 36, 1279–1297. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, T.P.; Ryabinkin, A.V. Zooplankton and macrozoobenthos in small reservoirs in different types of landscapes in southern Karelia. Proc. Karelian Res. Centre RAS 2015, 6, 47–63. (In Russian) [Google Scholar] [CrossRef]
- Rautio, M.; Sorvari, S.; Korhola, A. Diatom and crustacean zooplankton communities, their seasonal variability and representation in the sediments of subarctic Lake Saanajarvi. J. Limnol. 2000, 59, 81–96. [Google Scholar] [CrossRef]
- Krupa, E.; Romanova, S.; Berkinbaev, G.; Yakovleva, N.; Sadvakasov, E. Zooplankton as indicator of the ecological state of protected aquatic ecosystems (Lake Borovoe, Burabay National Nature Park, Northern Kazakhstan). Water 2020, 12, 2580. [Google Scholar] [CrossRef]
- Simoncelli, S.; Thackeray, S.J.; Wain, D.J. Effect of temperature on zooplankton vertical migration velocity. Hydrobiologia 2019, 829, 143–166. [Google Scholar] [CrossRef] [Green Version]
- Larsen, P.S.; Madsen, C.V.; Riisgård, H.U. Effect of temperature and viscosity on swimming velocity of the copepod Acartia tonsa, brine shrimp Artemia salina and rotifer Brachionus plicatilis. Aquat. Biol. 2008, 4, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Frolov, A.A. Ichthyofauna. In Engineering and Environmental Research for the Object Center for Construction of Large-Capacity Marine Structures (CSCMS) in the Village Area Belokamenka. Volume 2 (Lake Kulonga); Ishkulov, D.G., Frolov, A.A., Eds.; MMBI KSC RAS Press: Murmansk, Russia, 2016; pp. 47–63. (In Russian) [Google Scholar]
- Cohen, J.H.; Forward, R.B. Zooplankton diel vertical migration—A review of proximate control. Oceanogr. Mar. Biol. Ann. Rev. 2009, 47, 77–110. [Google Scholar]




| St. | Date | Coordinates | Local Time | Depth, m | Snow Thickness, m | Ice Thickness, m | Mean Water Temperature, °C |
|---|---|---|---|---|---|---|---|
| 2 | 3 March 2016 | 69°07.535′ N 33°05.115′ E | 12:07 | 24 | 0.20 | 0.62 | 3.2 |
| 3 | 4 March 2016 | 69°06.991′ N 33°06.356′ E | 11:15 | 13 | 0.18 | 0.60 | 2.8 |
| 4 | 4 March 2016 | 69°06.774′ N 33°06.799′ E | 13:33 | 2 | 0.40 | 0.63 | 0.8 |
| 5 | 4 March 2016 | 69°06.353′ N 33°07.281′ E | 14:59 | 4 | 0.15 | 0.65 | 1.0 |
| Taxon | Station | |||
|---|---|---|---|---|
| 2 | 3 | 4 | 5 | |
| Cyclopoida | ||||
| Acanthocyclops vernalis | 120/6.4 | 50/2.4 | - | - |
| Cyclops scutifer | 120/13.6 | - | - | 67/5.7 |
| Cyclops strenuus | 40/5.3 | - | - | - |
| Cyclops vicinus | 80/7.2 | - | - | - |
| Cyclops spp. IV–V | 320/2.4 | 300/2.2 | 200/1.8 | 133/0.9 |
| Mesocyclops leuckarti | 280/5.1 | 100/2 | - | 200/4.2 |
| Macrocyclops albidus | 120/22.1 | 50/12.7 | - | - |
| Eucyclops serrulatus | 80/4.1 | 100/4.7 | - | - |
| Calanoida | ||||
| Eudiaptomus gracilis | 80/3.4 | 200/7.9 | - | - |
| Eudiaptomus graciloides | 80/3.1 | 50/1.8 | - | - |
| Taxa | Average Dissimilarity, % | Contribution, % |
|---|---|---|
| Eudiaptomus gracilis | 17.05 | 17.05 |
| Cyclops spp. IV–V | 15.96 | 33.02 |
| Mesocyclops leuckarti | 15.63 | 48.65 |
| Eucyclops serrulatus | 10.47 | 59.12 |
| Acanthocyclops vernalis | 9.12 | 68.65 |
| Macrocyclops albidus | 9.12 | 77.37 |
| Lake | Region | Coordinates | Dominating Taxa | Abundance, ind. m−3 | Biomass, g wet mass m−3 | Shannon Index | Reference |
|---|---|---|---|---|---|---|---|
| Summer | |||||||
| Imandra | Kola Peninsula, central part | 67°40′ N, 33°00′ E | Bosmina obtusirostris Daphnia cristata Holopedium gibberum Mesocyclops leuckarti Eudiaptomus gracilis | 69,000 | 0.4 | 1.7–2.9 | [15] |
| Verhniy Tsagajavr | Kola Peninsula, central part | 67°33′ N, 35°07′ E | Kellicottia longispina Bosmina obtusirostris Holopedium gibberum Daphnia cristata Eudiaptomus gracilis | 37,500 | 0.94 | 1.65 | [16] |
| Nizhniy Tsagajavr | Kola Peninsula, central part | 67°34′ N, 35°07′ E | Keratella cochlearis Bosmina obtusirostris Cyclops sp. | 2250 | 0.06 | 1.22 | [16] |
| Sharjavr | Kola Peninsula, central part | 67°33′ N, 35°8′ E | Keratella cochlearis Kellicottia longispina Daphnia cristata Eudiaptomus gracilis Cyclops sp. | 10,950 | 0.52 | 2.06 | [16] |
| Lastjavr | Kola Peninsula, central part | 67°31′ N, 35°10′ E | Polyarthra sp. Brachionus calyciflorus Keratella cochlearis Kellicottia longispina Holopedium gibberum Eudiaptomus gracilis Cyclops sp. | 27,000 | 0.52 | 2.05 | [16] |
| Goluboe | Kola Peninsula, central part | 67°27′ N, 35°07′ E | Keratella cochlearis Kellicottia longispina Holopedium gibberum Bosmina obtusirostris Eudiaptomus gracilis Cyclops sp. | 14,080 | 0.32 | 2.48 | [16] |
| Verhne-Panskoe | Kola Peninsula, central part | 67°26′ N, 35°11′ E | Kellicottia longispina Bosmina obtusirostris Cyclops sp. | 1500 | 0.04 | 1.98 | [16] |
| Ryashkovskoye | Kola Peninsula, western part | 67°00′ N, 32°32′ E | Keratellacochlearis Kellicottialongispina Bosminaobtusirostris Eudiaptomusgraciloides | 5000–16,000 | 0.1–0.53 | 1.05–1.99 | [13] |
| Munozero | Karelia | 62°14′ N, 33°49′ E | Thermocyclops oithonoides Daphnia cristata Bosmina cf. coregoni Asplanchna sp. | 31,540–120,700 | 0.854–32.46 | 2.18–2.8 | [32] |
| Miagrozero | Karelia | 62°28′ N, 34°49′ E | Eudiaptomus gracilis Thermocyclops oithonoides Heterocope appendiculata Polyphemus pediculus Kellicottia longispina | 14,100 | 0.47 | 2.18 | [33] |
| Lelikozero | Karelia | 62°18′ N, 34°55′ E | Eudiaptomus gracilis Heterocope appendiculata Kellicottia longispina Bosmina obt. lacustris | 12,700 | 0.32 | 2.53 | [33] |
| Uros | Karelia | 62°16′ N, 33°12′ E | Eudiaptomus gracilis Holopedium gibberum Diaphanosoma brachyurum Bosmina longirostris Kellicottia longispina | 15,400 | 0.38 | 2.27 | [33] |
| Kondozero | Karelia | 62°31′ N, 34°14′ E | Eudiaptomus gracilis Kellicottia longispina Asplanchna priodonta | 6900 | 0.08 | 0.89 | [33] |
| Koverjarvi | Karelia | 61°34′ N, 33°23′ E | Kellicottia longispina Asplanchna priodonta Daphnia cristata Bosmina obt. lacustris Diaphanosoma brachyurum | 24,100 | 1.04 | 2.92 | [33] |
| Mikkelskoe | Karelia | 61°43′ N, 33°01′ E | Mesocyclops oithonoides Chydorus sphaericus Bosmina obt. lacustris Bosmina gibbera Daphnia cristata | 49,000–88,000 | 1.5–1.6 | – | [21] |
| Kroshnozero | Karelia | 61°40′ N, 33°07′ E | Mesocyclops oithonoides Mesocyclops leuckarti Bosmina obt. lacustris Daphnia cristata Chydorus sphaericus | 86,200–110,000 | 1.5–1.7 | – | [21] |
| Onego | Karelia | 61°46′ N, 34°30′ E | Limnocalanus macrurus Bosmina longispina Daphnia cristata Kellicottia longispina Asplanchna sp. | 5600 | 0.21 | – | [11] |
| Winter | |||||||
| Mikkelskoe | Karelia | 61°43′ N, 33°01′ E | Eudiaptomus gracilis Cyclops sp. Daphnia cristata Asplanchna priodonta | 2818 | 0.02–0.06 | – | [21] |
| Kroshnozero | Karelia | 61°40′ N, 33°07′ E | Eudiaptomus gracilis Eudiaptomus graciloides Daphnia cristata Notholca longispina | 4635–5120 | 0.1–0.2 | – | [21] |
| Onego | Karelia | 61°46′ N, 34°30′ E | Limnocalanus macrurus Eudiaptomus gracilis Megacyclops gigas | 500–700 | 0.012–0.018 | – | [11] |
| Kulonga | Kola Peninsula, northern part | 69°06′ N, 33°06′ E | Cyclops spp. Macrocyclops albidus Cyclops scutifer | 200–1320 | 0.018–0.073 | 1.77 | Our data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvoretsky, V.G.; Dvoretsky, A.G. Winter Zooplankton in a Small Arctic Lake: Abundance and Vertical Distribution. Water 2021, 13, 912. https://doi.org/10.3390/w13070912
Dvoretsky VG, Dvoretsky AG. Winter Zooplankton in a Small Arctic Lake: Abundance and Vertical Distribution. Water. 2021; 13(7):912. https://doi.org/10.3390/w13070912
Chicago/Turabian StyleDvoretsky, Vladimir G., and Alexander G. Dvoretsky. 2021. "Winter Zooplankton in a Small Arctic Lake: Abundance and Vertical Distribution" Water 13, no. 7: 912. https://doi.org/10.3390/w13070912