Hotspots of Legacy Phosphorus in Agricultural Landscapes: Revisiting Water-Extractable Phosphorus Pools in Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Characteristics
2.2. Soil Collection
2.3. Sample Extractions
2.4. Sample Analysis
2.5. Statistical Analysis
3. Results and Discussion
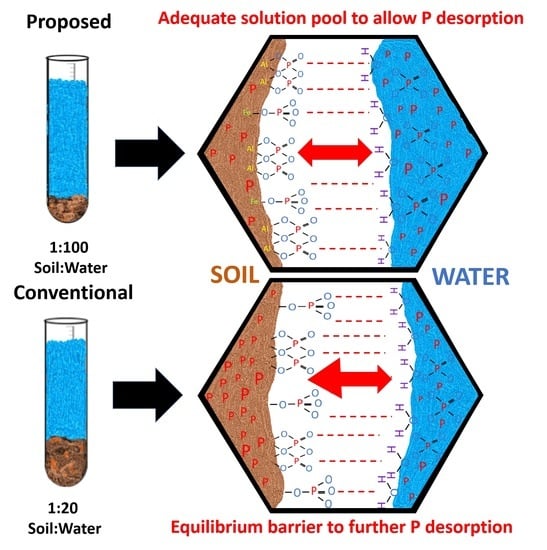
3.1. Water-Extractable Phosphorus across Extraction Ratios
3.2. Water-Extractable Phosphorus as a Proportion of Mehlich 3–Phosphorus and Total Phosphorus
3.3. Sequential Water Extractions to Exhaust Water-Extractable Phosphorus
3.4. Impact of Phosphorus Saturation on the Magnitude of Water-Extractable Phosphorus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Extraction Ratio | Site 1 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | |
|---|---|---|---|---|---|---|---|---|---|
| ––––––––––––––––––––––––mg kg−1––––––––––––––––––––––––––– | |||||||||
| WEPt | 1:10 | 18.4 | 54.0 | 34.4 | 34.7 | 25.4 | 29.1 | 23.0 | 21.4 |
| 1:20 | 24.3 | 70.4 | 43.6 | 40.8 | 38.2 | 38.9 | 32.6 | 31.3 | |
| 1:50 | 33.7 | 91.7 | 56.6 | 52.1 | 51.1 | 50.0 | 35.3 | 38.4 | |
| 1:100 | 41.7 | 132.2 | 68.0 | 63.0 | 66.1 | 52.7 | 45.3 | 36.5 | |
| WEPi | 1:10 | 14.8 | 44.7 | 26.8 | 27.2 | 19.8 | 22.1 | 17.6 | 16.8 |
| 1:20 | 22.9 | 56.5 | 35.5 | 33.4 | 29.9 | 30.1 | 24.2 | 28.2 | |
| 1:50 | 28.4 | 76.5 | 52.1 | 47.9 | 47.2 | 44.2 | 31.9 | 34.9 | |
| 1:100 | 38.5 | 121.1 | 62.9 | 57.8 | 61.5 | 49.2 | 43.1 | 34.2 | |
| WEPo | 1:10 | 3.5 | 9.2 | 7.6 | 7.5 | 5.6 | 7.0 | 5.3 | 4.6 |
| 1:20 | 1.4 | 13.9 | 8.2 | 7.4 | 8.3 | 8.8 | 8.5 | 3.1 | |
| 1:50 | 5.3 | 15.2 | 4.4 | 4.3 | 3.9 | 5.8 | 3.4 | 3.5 | |
| 1:100 | 3.2 | 11.0 | 5.0 | 5.2 | 4.5 | 3.5 | 2.2 | 2.4 | |
| Extraction Ratio | Site 1 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | |
|---|---|---|---|---|---|---|---|---|---|
| ––––––––––––––––––––––––% of Mehlich 3–P–––––––––––––– | |||||||||
| WEPt:M3–P | 1:10 | 3.4 | 4.8 | 4.3 | 5.0 | 5.1 | 3.7 | 3.8 | 3.4 |
| 1:20 | 4.6 | 6.2 | 5.4 | 5.9 | 7.6 | 5.0 | 5.4 | 5.0 | |
| 1:50 | 6.3 | 8.1 | 7.0 | 7.5 | 10.2 | 6.4 | 5.9 | 6.2 | |
| 1:100 | 7.8 | 11.7 | 8.4 | 9.1 | 13.2 | 6.7 | 7.5 | 5.9 | |
| WEPi:M3–P | 1:10 | 2.8 | 4.0 | 3.3 | 3.9 | 4.0 | 2.8 | 2.9 | 2.7 |
| 1:20 | 4.3 | 5.0 | 4.4 | 4.8 | 6.0 | 3.8 | 4.0 | 4.6 | |
| 1:50 | 5.3 | 6.8 | 6.5 | 6.9 | 9.4 | 5.6 | 5.3 | 5.6 | |
| 1:100 | 7.2 | 10.7 | 7.8 | 8.3 | 12.3 | 6.3 | 7.2 | 5.5 | |
| WEPo:M3–P | 1:10 | 0.7 | 0.8 | 0.9 | 1.1 | 1.1 | 0.9 | 0.9 | 0.7 |
| 1:20 | 0.3 | 1.2 | 1.0 | 1.1 | 1.6 | 1.1 | 1.4 | 0.5 | |
| 1:50 | 1.0 | 1.3 | 0.6 | 0.6 | 0.8 | 0.7 | 0.6 | 0.6 | |
| 1:100 | 0.6 | 1.0 | 0.6 | 0.8 | 0.9 | 0.4 | 0.4 | 0.4 | |
| Extraction Ratio | Site 1 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 | |
|---|---|---|---|---|---|---|---|---|---|
| –––––––––––––––––––––% of total P–––––––––––––––––––––– | |||||||||
| WEPt:TP | 1:10 | 2.7 | 2.4 | 2.4 | 2.5 | 1.8 | 2.1 | 2.8 | 2.9 |
| 1:20 | 3.5 | 3.2 | 3.0 | 2.9 | 2.6 | 2.8 | 3.9 | 4.2 | |
| 1:50 | 4.9 | 4.1 | 3.9 | 3.7 | 3.5 | 3.6 | 4.2 | 5.1 | |
| 1:100 | 6.0 | 5.9 | 4.7 | 4.5 | 4.6 | 3.7 | 5.4 | 4.9 | |
| WEPi:TP | 1:10 | 2.1 | 2.0 | 1.8 | 1.9 | 1.4 | 1.6 | 2.1 | 2.2 |
| 1:20 | 3.3 | 2.5 | 2.4 | 2.4 | 2.1 | 2.1 | 2.9 | 3.8 | |
| 1:50 | 4.1 | 3.4 | 3.6 | 3.4 | 3.3 | 3.1 | 3.8 | 4.7 | |
| 1:100 | 5.6 | 5.4 | 4.3 | 4.1 | 4.3 | 3.5 | 5.2 | 4.6 | |
| WEPo:TP | 1:10 | 0.5 | 0.4 | 0.5 | 0.5 | 0.4 | 0.5 | 0.6 | 0.6 |
| 1:20 | 0.2 | 0.6 | 0.6 | 0.5 | 0.6 | 0.6 | 1.0 | 0.4 | |
| 1:50 | 0.8 | 0.7 | 0.3 | 0.3 | 0.3 | 0.4 | 0.4 | 0.5 | |
| 1:100 | 0.5 | 0.5 | 0.3 | 0.4 | 0.3 | 0.2 | 0.3 | 0.3 | |
| Sequential Extraction Number | Site 2 1 | Site 4 | Site 6 | Site 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WEPt | WEPi | WEPo | WEPt | WEPi | WEPo | WEPt | WEPi | WEPo | WEPt | WEPi | WEPo | |
| ––––––––––––––––––––––––––––––––––––––––– mg L−1 ––––––––––––––––––––––––––––––––– | ||||||||||||
| 1 | 1.34 | 1.23 | 0.11 | 0.65 | 0.59 | 0.05 | 0.53 | 0.50 | 0.03 | 0.46 | 0.44 | 0.02 |
| 2 | 0.85 | 0.79 | 0.06 | 0.32 | 0.29 | 0.03 | 0.25 | 0.24 | 0.01 | 0.22 | 0.21 | 0.01 |
| 3 | 0.68 | 0.63 | 0.05 | 0.26 | 0.25 | 0.01 | 0.18 | 0.18 | 0.00 | 0.15 | 0.15 | 0.01 |
| 4 | 0.59 | 0.56 | 0.03 | 0.21 | 0.21 | 0.00 | 0.18 | 0.18 | 0.01 | 0.12 | 0.11 | 0.01 |
| 5 | 0.52 | 0.47 | 0.05 | 0.22 | 0.21 | 0.01 | 0.17 | 0.17 | 0.00 | 0.10 | 0.09 | 0.00 |
| 6 | 0.55 | 0.53 | 0.02 | 0.23 | 0.23 | 0.00 | 0.13 | 0.14 | 0.00 | 0.07 | 0.07 | −0.01 |
| 7 | 0.54 | 0.51 | 0.03 | 0.20 | 0.19 | 0.01 | 0.15 | 0.14 | 0.00 | 0.07 | 0.07 | 0.00 |
| 8 | 0.49 | 0.46 | 0.03 | 0.18 | 0.18 | 0.00 | 0.14 | 0.14 | 0.00 | 0.08 | 0.08 | 0.00 |
| Sequential Extraction Number | Site 2 1 | Site 4 | Site 6 | Site 7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WEPt | WEPi | WEPo | WEPt | WEPi | WEPo | WEPt | WEPi | WEPo | WEPt | WEPi | WEPo | |
| –––––––––––––––––––––––––––––––––––––––– mg kg−1 –––––––––––––––––––––––––––––––– | ||||||||||||
| 1 | 132.2 | 121.1 | 11.0 | 63.0 | 57.8 | 5.2 | 52.7 | 49.2 | 3.5 | 45.3 | 43.1 | 2.2 |
| 2 | 84.0 | 77.9 | 6.2 | 32.0 | 29.3 | 2.6 | 24.7 | 23.9 | 0.8 | 21.5 | 20.9 | 0.7 |
| 3 | 67.5 | 62.7 | 4.8 | 25.5 | 24.7 | 0.8 | 17.8 | 17.4 | 0.3 | 15.2 | 14.4 | 0.7 |
| 4 | 58.3 | 54.9 | 3.4 | 20.9 | 20.4 | 0.5 | 18.0 | 17.5 | 0.5 | 12.1 | 11.2 | 0.9 |
| 5 | 51.0 | 46.1 | 4.9 | 21.4 | 20.3 | 1.2 | 17.1 | 16.7 | 0.4 | 9.5 | 9.4 | 0.2 |
| 6 | 54.7 | 52.3 | 2.4 | 22.8 | 22.9 | −0.1 | 13.3 | 13.5 | −0.2 | 6.6 | 7.2 | −0.6 |
| 7 | 53.2 | 50.1 | 3.1 | 19.9 | 18.9 | 1.0 | 14.5 | 14.1 | 0.3 | 7.0 | 7.0 | 0.0 |
| 8 | 48.4 | 45.0 | 3.4 | 17.6 | 17.8 | −0.2 | 13.8 | 14.2 | −0.4 | 7.6 | 7.7 | 0.0 |
| Cumulative (1–8) | 549.3 | 510.1 | 39.3 | 223.1 | 212.1 | 11.0 | 171.8 | 166.6 | 5.2 | 124.9 | 120.8 | 4.1 |

References
- Kleinman, P.J.A.; Fanelli, R.M.; Hirsch, R.M.; Buda, A.R.; Easton, Z.M.; Wainger, L.A.; Brosch, C.; Lowenfish, M.; Collick, A.S.; Shirmohammadi, A.; et al. Phosphorus and the Chesapeake Bay: Lingering Issues and Emerging Concerns for Agriculture. J. Environ. Qual. 2019, 48, 1191–1203. [Google Scholar] [CrossRef] [Green Version]
- Kusmer, A.S.; Goyette, J.-O.; Macdonald, G.K.; Bennett, E.M.; Maranger, R.; Withers, P.J.A. Watershed Buffering of Legacy Phosphorus Pressure at a Regional Scale: A Comparison Across Space and Time. Ecosystems 2019, 22, 91–109. [Google Scholar] [CrossRef] [Green Version]
- Toor, G.S.; Sims, J.T. Managing Phosphorus Leaching in Mid-Atlantic Soils: Importance of Legacy Sources. Vadose Zone J. 2015, 14. [Google Scholar] [CrossRef]
- Toor, G.S.; Yang, Y.; Morris, M.; Schwartz, P.; Darwish, Y.; Gaylord, G.; Webb, K. Phosphorus Pools in Soils Under Rotational and Continuous Grazed Pastures. Agrosyst. Geosci. Environ. 2020, 3, 20103. [Google Scholar] [CrossRef]
- Daniel, T.C.; Sharpley, A.N.; Edwards, D.R.; Wedepohl, R.; Lemunyon, J.L. Minimizing Surface Water Eutrophication from Agriculture by Phosphorus Management. J. Soil Water Conserv. 1994, 49, 30. [Google Scholar]
- Carpenter, S.R. Phosphorus Control is Critical to Mitigating Eutrophication. Proc. Natl. Acad. Sci. USA 2008, 105, 11039–11040. [Google Scholar] [CrossRef] [Green Version]
- Osterholz, W.R.; Hanrahan, B.R.; King, K.W. Legacy Phosphorus Concentration–Discharge Relationships in Surface Runoff and Tile Drainage from Ohio Crop Fields. J. Environ. Qual. 2020, 49, 675–687. [Google Scholar] [CrossRef]
- Toor, G.S.; Sims, J.T. Phosphorus Leaching in Soils Amended with Animal Manures Generated from Modified Diets. J. Environ. Qual. 2016, 45, 1385–1391. [Google Scholar] [CrossRef]
- Toor, G.S.; Sims, J.T. Managing Legacy and New Sources of Phosphorus to Reduce Leaching in Mid-Atlantic Soils. Crop. Soils 2016, 49, 40–47. [Google Scholar] [CrossRef]
- Kleinman, P.J.; Allen, A.L.; Needelman, B.A.; Sharpley, A.N.; Vadas, P.A.; Saporito, L.S.; Folmar, G.J.; Bryant, R.B. Dynamics of Phosphorus Transfers from Heavily Manured Coastal Plain Soils to Drainage Ditches. J. Soil Water Conserv. 2007, 62, 225–235. [Google Scholar]
- Sharpley, A.N.; Weld, J.L.; Beegle, D.B.; Kleinman, P.J.; Gburek, W.J.; Moore, P.A.; Mullins, G. Development of Phosphorus Indices for Nutrient Management Planning Strategies in the United States. J. Soil Water Conserv. 2003, 58, 137–152. [Google Scholar]
- Osmond, D.L.; Cabrera, M.L.; Feagley, S.E.; Hardee, G.E.; Mitchell, C.C.; Moore, P.A.; Mylavarapu, R.S.; Oldham, J.L.; Stevens, J.C.; Thom, W.O.; et al. Comparing Ratings of the Southern Phosphorus Indices. J. Soil Water Conserv. 2006, 61, 325–337. [Google Scholar]
- Maryland Department of Agriculture. Soils Data Collection and Verification as of January 2019; Maryland Department of Agriculture: Annapolis, MD, USA, 2019.
- Sharpley, A.N. Dependence of Runoff Phosphorus on Extractable Soil Phosphorus. J. Environ. Qual. 1995, 24, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Pote, D.H.; Daniel, T.C.; Moore, P.A.; Nichols, D.J.; Sharpley, A.N.; Edwards, D.R. Relating Extractable Soil Phosphorus to Phosphorus Losses in Runoff. Soil Sci. Soc. Am. J. 1996, 60, 855–859. [Google Scholar] [CrossRef]
- Hooda, P.S.; Rendell, A.R.; Edwards, A.C.; Withers, P.J.A.; Aitken, M.N.; Truesdale, V.W. Relating Soil Phosphorus Indices to Potential Phosphorus Release to Water. J. Environ. Qual. 2000, 29, 1166–1171. [Google Scholar] [CrossRef] [Green Version]
- Maguire, R.O.; Sims, J.T. Soil Testing to Predict Phosphorus Leaching. J. Environ. Qual. 2002, 31, 1601–1609. [Google Scholar] [CrossRef]
- Self-Davis, M.L.; Moore, P.A., Jr.; Joern, B.C. Determination of Water- and/or Dilute Salt-extractable Phosphorus. In Methods Phosphorus Analysis for Soils, Sediments, Residuals, and Waters; Southern Cooperative Series Bulletin No. #396; North Carolina State University: Raleigh, NC, USA, 2000; pp. 24–26. [Google Scholar]
- Beauchemin, S.; Hesterberg, D.; Chou, J.; Beauchemin, M.; Simard, R.R.; Sayers, D.E. Speciation of Phosphorus in Phosphorus-Enriched Agricultural Soils Using X-Ray Absorption Near-Edge Structure Spectroscopy and Chemical Fractionation. J. Environ. Qual. 2003, 32, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Sharpley, A.N.; McDowell, R.W.; Kleinman, P.J.A. Amounts, Forms, and Solubility of Phosphorus in Soils Receiving Manure. Soil Sci. Soc. Am. J. 2004, 68, 2048–2057. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, P.; Sullivan, D.; Wolf, A.; Brandt, R.; Dou, Z.; Elliott, H.; Kovar, J.; Leytem, A.; Maguire, R.; Moore, P.; et al. Selection of a Water-Extractable Phosphorus Test for Manures and Biosolids as an Indicator of Runoff Loss Potential. J. Environ. Qual. 2007, 36, 1357–1367. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, P.J.A.; Sharpley, A.N.; Wolf, A.M.; Beegle, D.B.; Moore, P.A. Measuring Water-Extractable Phosphorus in Manure as an Indicator of Phosphorus in Runoff. Soil Sci. Soc. Am. J. 2002, 66, 2009–2015. [Google Scholar] [CrossRef] [Green Version]
- Toor, G.S.; Haggard, B.E.; Reiter, M.S.; Daniel, T.C.; Donoghue, A.M. Phosphorus Solubility in Poultry Litters and Granulates: Influence of Litter Treatments and Extraction Ratios. Trans. ASABE 2007, 50, 533–542. [Google Scholar] [CrossRef]
- Sharpley, A.N. Depth of Surface Soil-runoff Interaction as Affected by Rainfall, Soil Slope, and Management. Soil Sci. Soc. Am. J. 1985, 49, 1010–1015. [Google Scholar] [CrossRef] [Green Version]
- Vadas, P.A.; Mallarino, A.P.; McFarland, A. The Importance of Sampling Depth When Testing Soils for their Potential to Supply Phosphorus to Surface Runoff. In Extension Fact Sheets; 2005; p. 14. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=188150 (accessed on 6 April 2021).
- Murphy, J.; Riley, J. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Gee, G.W.; Orr, D. 2.4 Particle-Size Analysis. Methods Soil Anal. 2002, 5, 255–293. [Google Scholar]
- Wolf, A.M.; Beegle, D.B. Recommended Soil Tests for Macronutrients. Recomm. Soil Test. Proced. Northeast. USA 2011, 493, 39–47. [Google Scholar]
- USEPA. Method 3050B Acid Digestion of Sediments, Sludges, and Soils; Revision 2; USEPA: Washington, DC, USA, 1996; pp. 1–12. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/epa-3050b.pdf (accessed on 6 April 2021).
- Fuhrman, J.K.; Zhang, H.; Schroder, J.L.; Davis, R.L.; Payton, M.E. Water-Soluble Phosphorus as Affected by Soil to Extractant Ratios, Extraction Times, and Electrolyte. Commun. Soil Sci. Plant Anal. 2005, 36, 925–935. [Google Scholar] [CrossRef]
- Sharpley, A.N. Effect of Soil Properties on the Kinetics of Phosphorus Desorption. Soil Sci. Soc. Am. J. 1983, 47, 462–467. [Google Scholar] [CrossRef]
- Leytem, A.B.; Mikkelsen, R.L.; Gilliam, J.W. Sorption of Organic Phosphorus Compounds in Atlantic Coastal Plain Soils. Soil Sci. 2002, 167, 652–658. [Google Scholar] [CrossRef] [Green Version]
- Waldrip, H.M.; Pagliari, P.H.; He, Z.; Harmel, R.D.; Cole, N.A.; Zhang, M. Legacy Phosphorus in Calcareous Soils: Effects of Long-Term Poultry Litter Application. Soil Sci. Soc. Am. J. 2015, 79, 1601–1614. [Google Scholar] [CrossRef]
- Koopmans, G.F.; Chardon, W.J.; McDowell, R.W. Phosphorus Movement and Speciation in a Sandy Soil Profile after Long-Term Animal Manure Applications. J. Environ. Qual. 2007, 36, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Dodd, R.; Sharpley, A. Recognizing the Role of Soil Organic Phosphorus in Soil Fertility and Water Quality. Resour. Conserv. Recycl. 2015, 105, 282–293. [Google Scholar] [CrossRef]
- USEPA. Ambient Water Quality Criteria Recommendations Information Supporting the Development of State and Tribal Nutrient Criteria Rivers and Streams in Nutrient Ecoregion XIV; EPA 822-B-00-022; USEPA: Washington, DC, USA, 2000. [Google Scholar]
- Nair, V.D.; Portier, K.M.; Graetz, D.A.; Walker, M.L. An Environmental Threshold for Degree of Phosphorus Saturation in Sandy Soils. J. Environ. Qual. 2004, 33, 107–113. [Google Scholar] [CrossRef]
- Sims, J.T.; Maguire, R.O.; Leytem, A.B.; Gartley, K.L.; Pautler, M.C. Evaluation of Mehlich 3 as an Agri-Environmental Soil Phosphorus Test for the Mid-Atlantic United States of America. Soil Sci. Soc. Am. J. 2002, 66, 2016–2032. [Google Scholar] [CrossRef]
- Dari, B.; Nair, V.D.; Sharpley, A.N.; Kleinman, P.; Franklin, D.; Harris, W.G. Consistency of the Threshold Phosphorus Saturation Ratio across a Wide Geographic Range of Acid Soils. Agrosyst. Geosci. Environ. 2018, 1, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Lucas, E.R.; Toor, G.S.; McGrath, J.M. Agronomic and Environmental Phosphorus Decline in Coastal Plain Soils after Cessation of Manure Application. Agric. Ecosyst. Environ. 2021, 311, 107337. [Google Scholar] [CrossRef]



| Soil Characteristics | Site 1 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | Site 8 |
|---|---|---|---|---|---|---|---|---|
| pH | 6.55 | 7.25 | 6.56 | 6.36 | 6.14 | 6.22 | 6.35 | 6.38 |
| Organic Matter (%) | 0.56 | 1.87 | 1.87 | 2.11 | 2.89 | 2.08 | 1.00 | 0.99 |
| CEC (meq/100 g) | 6.40 | 15.10 | 11.70 | 12.50 | 13.20 | 10.20 | 5.90 | 8.30 |
| % Sand | 77.7 | 53.9 | 67.7 | 51.3 | 56.9 | 53.6 | 85.0 | 86.1 |
| % Silt | 17.5 | 40.6 | 23.7 | 39.2 | 32.7 | 38.6 | 12.1 | 11.4 |
| % Clay | 4.8 | 5.5 | 8.6 | 9.5 | 10.4 | 7.8 | 3.0 | 2.5 |
| Texture | Loamy Sand | Sandy Loam | Sandy Loam | Loam | Sandy Loam | Sandy Loam | Loamy Sand | Loamy Sand |
| USDA-NRCS soil classification | Coarse-loamy, siliceous, semiactive, mesic Typic Hapludults | Fine-loamy, mixed, active, mesic Typic Endoaquults | Fine-loamy, mixed, semiactive, mesic Typic Hapludults | Fine-loamy, mixed, active, mesic Typic Endoaquults | Fine-loamy, mixed, active, mesic Typic Endoaquults | Fine-loamy, mixed, active, mesic Typic Endoaquults | Coarse-loamy, siliceous, semiactive, mesic Aquic Hapludults | Coarse-loamy, siliceous, semiactive, mesic Aquic Hapludults |
| WRB Soil Classification | Acrisol | Gleysol | Acrisol | Gleysol | Gleysol | Gleysol | Acrisol | Acrisol |
| Acidity (meq/100 g) | 2.0 | 0.0 | 2.8 | 2.8 | 3.9 | 2.8 | 2.0 | 2.0 |
| Total P (mg/kg) | 691.9 | 2235.2 | 1460.7 | 1401.1 | 1446.4 | 1405.4 | 832.4 | 749.4 |
| M3–P (mg/kg) | 534.0 | 1127.0 | 806.0 | 692.0 | 502.0 | 785.0 | 602.0 | 620.0 |
| M3–Fe (mg/kg) | 281.6 | 270.5 | 357.8 | 316.7 | 275.6 | 183.0 | 185.4 | 164.1 |
| M3–Al (mg/kg) | 789.1 | 586.4 | 784.4 | 586.7 | 905.0 | 1006.0 | 767.3 | 615.1 |
| M3–PSR | 0.50 | 1.37 | 0.73 | 0.81 | 0.42 | 0.62 | 0.61 | 0.78 |
| M3–P:Total P | 0.77 | 0.50 | 0.55 | 0.49 | 0.35 | 0.56 | 0.72 | 0.83 |
| M3–Ca (mg/kg) | 721.4 | 2662.4 | 1375.1 | 1605.3 | 1495.1 | 1203.7 | 591.7 | 995.3 |
| M3–Mg (mg/kg) | 58.0 | 167.0 | 180.0 | 162.0 | 158.0 | 104.0 | 89.0 | 111.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roswall, T.; Lucas, E.; Yang, Y.-Y.; Burgis, C.; Scott, I.S.P.C.; Toor, G.S. Hotspots of Legacy Phosphorus in Agricultural Landscapes: Revisiting Water-Extractable Phosphorus Pools in Soils. Water 2021, 13, 1006. https://doi.org/10.3390/w13081006
Roswall T, Lucas E, Yang Y-Y, Burgis C, Scott ISPC, Toor GS. Hotspots of Legacy Phosphorus in Agricultural Landscapes: Revisiting Water-Extractable Phosphorus Pools in Soils. Water. 2021; 13(8):1006. https://doi.org/10.3390/w13081006
Chicago/Turabian StyleRoswall, Taylor, Emileigh Lucas, Yun-Ya Yang, Charles Burgis, Isis S.P.C. Scott, and Gurpal S. Toor. 2021. "Hotspots of Legacy Phosphorus in Agricultural Landscapes: Revisiting Water-Extractable Phosphorus Pools in Soils" Water 13, no. 8: 1006. https://doi.org/10.3390/w13081006