Hydrochemical Zoning and Chemical Evolution of the Deep Upper Jurassic Thermal Groundwater Reservoir Using Water Chemical and Environmental Isotope Data
Abstract
:1. Introduction
2. Geological and Hydrogeological Setting

- It was assumed that the recharge areas of the Upper Jurassic groundwater are located at the north-western boundary of the SGMB in the Swabian Alb. The general flow regime was determined by hydraulic potential analysis to be along the river Danube with a flow direction to the east and in direction to the central SGMB [25,36,37]. Observed higher mineralised ion-exchange waters at the north-eastern margin showed some evidence of groundwater flow from the central basin to the north-east of the SGMB [34,35];
- The connate Upper Jurassic formation water was washed out of the aquifer. The low mineralised groundwater in the central SGMB was believed to be a mixture of meteoric water and higher mineralised formation or oil field waters, presumably seeping from the overlying Tertiary sediments, which are responsible for the higher and dominating amounts of sodium and chloride [2,24,25,29,31,33,37,38,56]. Subsequently, on the basis of the assumed geochemical evolution of the Upper Jurassic groundwater in the central SGMB, it was assumed that the groundwater flows from west to south-east towards the Alps [37];
- The concept of subglacial recharge and cross-formational flow in the south-west of the SGMB (lake Constance region) and ion-exchange of paleo-water with an assumed northwards flow direction to the draining river Danube by [32,57] was recently supported by groundwater dating results derived from Kr [44] and C [58]. Based on these results, recharge areas for the Upper Jurassic groundwater in the central SGMB were postulated to be at the northern fringe of the Alps, or south of the Northern Calcareous Alps [26].
3. Materials and Methods
3.1. Groundwater Sampling and Analysis
3.1.1. Water Chemical Parameters
3.1.2. Stable Water Isotopes
3.1.3. Strontium Isotopes
3.1.4. Noble Gases, He/He and Ar/Ar
3.2. Calculation of Noble Gas Infiltration Temperatures (NGTs) Using Ne, Kr and Xe
3.3. Determination of Apparent Mean Residence Times with He and Ar
3.4. Multivariate Statistical Techniques
3.4.1. Hierarchical Cluster Analysis HCA
3.4.2. Exploratory Factor Analysis EFA
4. Results and Discussion
4.1. Results of Multivariate Statistical Analyses HCA and EFA
4.1.1. Classification of Different Water Types of the Upper Jurassic Reservoir in the SGMB Based on HCA
4.1.2. Identification of Factors and Hydrogeological Processes Affecting the Hydrochemical Water Composition
4.2. Chemical Analyses of Grouped Water Types
4.2.1. Water Type 1
4.2.2. Water Type 2
4.2.3. Water Type 3
4.3. Assessing Recharge Conditions and Water Rock Interaction
4.3.1. Noble Gas Infiltration Temperatures NGTs and Stable Water Isotopes
4.3.2. Tracing Water-Rock Interaction with Sr/Sr Signatures
4.3.3. Mixing Processes and Origin of Salinity Using δO and Cl
4.4. Calculation of Apparent Water Ages by Radiogenic Noble Gas Isotopes
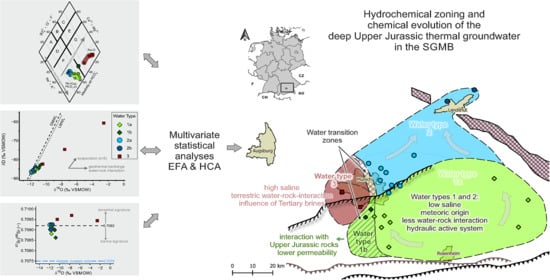
4.5. Regional Linking of Water Type Classification and Hydrogeochemical Genesis of the Upper Jurassic Reservoir
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Results of Data Measurements
| ID | pH | EC | Ca | Mg | Na | K | Li | Sr | Rb | HCO | Cl | SO | F | Br | DOC | B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (-) | (µS/cm) | (mg/L) | ||||||||||||||
| 1 | 7.1 | 715 | 31.8 | 9.4 | 121.9 | 15.2 | 0.12 | 0.57 | 0.022 | 329 | 70.0 | 1.6 | 2.4 | 0.38 | 0.7 | 2.3 |
| 2 | 6.9 | 756 | 38.5 | 10.7 | 129.8 | 14.5 | 0.20 | 0.71 | 0.034 | 354 | 75.6 | 13.4 | 5.1 | 0.22 | 0.8 | 0.7 |
| 3 | 6.8 | 723 | 35.3 | 10.2 | 120.0 | 12.8 | 0.11 | 0.61 | 0.035 | 333 | 72.6 | 6.4 | 2.8 | 1.27 | 1.0 | 0.6 |
| 4 | 7.0 | 722 | 31.9 | 8.9 | 123.8 | 7.3 | 0.14 | 0.65 | 0.023 | 345 | 70.4 | 2.9 | 1.8 | 0.43 | 1.2 | 2.5 |
| 5 | 7.1 | 740 | 35.1 | 10.4 | 122.0 | 13.8 | 0.10 | 0.56 | 0.033 | 323 | 69.8 | <1.6 | 2.7 | 0.27 | 0.9 | 0.7 |
| 6 | 7.1 | 700 | 27.1 | 7.2 | 122.5 | 14.8 | 0.11 | 0.65 | 0.036 | 329 | 69.5 | <1.6 | 1.9 | 0.39 | 0.8 | 0.8 |
| 7 | 6.9 | 697 | 28.6 | 8.8 | 120.9 | 14.3 | 0.10 | 0.57 | 0.033 | 305 | 69.9 | <1.6 | 3.3 | 0.43 | 0.5 | 0.8 |
| 8 | 6.9 | 700 | 30.4 | 7.5 | 122.3 | 14.7 | 0.12 | 0.57 | 0.036 | 302 | 82.5 | 1.4 | 2.2 | 0.39 | 1.7 | 2.1 |
| 9 | 7.2 | 752 | 33.7 | 10.7 | 120.0 | 14.1 | 0.16 | 0.57 | 0.022 | 326 | 72.0 | 7.8 | 6.6 | 0.42 | 0.9 | 2.2 |
| 10 | 6.5 | 587 | 3.5 | 1.6 | 130.1 | 17.2 | 0.10 | 0.16 | 0.053 | 214 | 74.0 | 4.1 | 3.0 | 0.33 | 1.6 | 1.1 |
| 11 | 6.3 | 600 | 8.8 | 1.0 | 118.5 | 15.9 | 0.12 | 0.28 | 0.059 | 207 | 73.8 | 5.3 | 3.6 | 0.40 | 2.6 | 0.8 |
| 12 | 6.5 | 653 | 16.9 | 1.7 | 121.6 | 19.8 | 0.14 | 0.75 | 0.063 | 256 | 69.1 | 4.8 | 2.0 | 0.19 | 1.7 | <0.3 |
| 13 | 6.7 | 699 | 23.4 | 3.1 | 131.0 | 21.2 | 0.16 | 0.59 | 0.059 | 275 | 81.7 | 8.2 | 4.2 | <0.1 | 2.0 | 2.2 |
| 14 | 6.3 | 689 | 16.6 | 1.8 | 134.7 | 22.9 | 0.17 | 0.50 | 0.067 | 250 | 82.2 | 15.8 | 5.1 | <0.1 | 1.5 | 2.1 |
| 15 | 6.4 | 746 | 18.5 | 2.1 | 143.5 | 20.6 | 0.19 | 0.55 | 0.066 | 275 | 95.8 | 13.9 | 7.9 | 0.55 | 2.3 | <0.3 |
| 16 | 6.7 | 681 | 24.8 | 3.7 | 121.6 | 15.8 | 0.13 | 0.65 | 0.043 | 281 | 77.1 | 4.3 | 4.6 | <0.1 | 1.6 | 2.2 |
| 17 | 6.5 | 713 | 27.0 | 3.8 | 129.1 | 18.9 | 0.15 | 0.99 | 0.053 | 288 | 79.0 | 12.5 | 2.7 | 0.33 | 1.9 | 2.2 |
| 18 | 6.4 | 1087 | 35.9 | 5.2 | 172.6 | 32.1 | 0.24 | 1.40 | 0.118 | 311 | 157.6 | 30.9 | 7.7 | 0.84 | 3.8 | 3.3 |
| 19 | 6.4 | 1029 | 38.2 | 4.2 | 174.0 | 31.3 | 0.27 | 1.09 | 0.094 | 311 | 157.1 | 20.6 | 4.5 | 0.55 | 4.2 | 3.0 |
| 20 | 6.9 | 1596 | 39.3 | 4.0 | 269.0 | 34.9 | 0.38 | 1.73 | 0.057 | 317 | 279.3 | 45.0 | 2.2 | 0.62 | 4.4 | 8.8 |
| 21 | 6.6 | 3800 | 91.6 | 13.9 | 792.1 | 51.8 | 0.92 | 4.02 | 0.015 | 500 | 968.9 | 100.6 | 5.1 | 3.19 | 21.1 | 15.8 |
| 22 | 6.6 | 7703 | 135.0 | 29.0 | 1854.0 | 85.2 | 1.91 | 6.40 | 0.150 | 854 | 2485.0 | 337.0 | 4.6 | 9.92 | 70.5 | 35.0 |
| 23 | 7.5 | 965 | 34.0 | 17.9 | 149.8 | 15.4 | 0.13 | 0.15 | 0.041 | 410 | 93.7 | 4.6 | 0.53 | 0.8 | 0.9 | |
| 24 | 7.0 | 820 | 43.6 | 10.2 | 137.4 | 15.9 | 0.10 | 0.20 | 0.039 | 412 | 79.7 | 0.3 | 2.7 | 0.40 | 0.5 | 0.7 |
| ID | δO | δD | Sr/Sr | He | He/He | Herad | Ar | Ar/Ar | Arrad |
|---|---|---|---|---|---|---|---|---|---|
| (‰ VSMOW) | (-) | (ccSTP/g) | (-) | (ccSTP/g) | (-) | (ccSTP/g) | |||
| 1 | −11.7 | −86.3 | 0.70921 | ||||||
| 2 | −11.7 | −86.2 | 0.70907 | 2.30 | 9.89 | 2.29 | |||
| 3 | −11.9 | −86.8 | 0.70925 | 2.22 | 1.01 | 2.21 | |||
| 4 | −11.6 | −85.4 | 0.70923 | ||||||
| 5 | −11.7 | −85.9 | 0.70922 | 1.80 | 1.12 | 1.79 | |||
| 6 | −11.7 | −86.2 | 0.70922 | 2.30 | 1.36 | 2.29 | |||
| 7 | −11.5 | −86.2 | 0.70922 | 2.32 | 1.06 | 2.31 | |||
| 8 | −12.0 | −86.3 | 0.70924 | ||||||
| 9 | −11.6 | −86.1 | 0.70926 | 2.31 | 1.20 | 2.30 | 5.03 | 302.5 | 6.65 |
| 10 | −11.6 | −86.0 | 0.70913 | ||||||
| 11 | −11.6 | −86.1 | 0.70917 | ||||||
| 12 | −11.6 | −86.5 | 0.70905 | 2.29 | 1.03 | 2.28 | |||
| 13 | −11.8 | −86.3 | 0.70901 | 2.42 | 9.79 | 2.41 | |||
| 14 | −11.4 | −85.1 | 0.70920 | ||||||
| 15 | −11.0 | −84.8 | 0.70902 | 1.68 | 9.07 | 1.67 | |||
| 16 | −11.7 | −86.0 | 0.70925 | 2.53 | 1.13 | 2.52 | 5.04 | 299.8 | 2.17 |
| 17 | −11.6 | −85.8 | 0.70881 | 2.42 | 1.10 | 2.42 | 4.95 | 301.2 | 4.39 |
| 18 | −10.8 | −82.8 | 0.70862 | 9.20 | 7.31 | 9.19 | 1.65 | 307.6 | 5.76 |
| 19 | −11.0 | −84.3 | 0.70899 | 4.22 | 6.45 | 4.21 | |||
| 20 | −10.5 | −82.3 | 0.70951 | 5.44 | 7.31 | 5.43 | |||
| 21 | −7.6 | −74.6 | 0.70970 | 1.15 | 6.26 | 1.15 | 1.98 | 345.1 | |
| 22 | −2.6 | −60.6 | 0.70944 | 1.00 | 8.08 | 1.00 | 9.39 | 392 | |
| 23 | −11.4 | −84.0 | 0.70926 | ||||||
| 24 | −11.7 | −85.7 | 0.70901 | ||||||
| ID | He | ΔHe | Ne | ΔNe | Ar | ΔAr | Kr | ΔKr | Xe | ΔXe |
|---|---|---|---|---|---|---|---|---|---|---|
| (ccSTP/g) | ||||||||||
| 1 | ||||||||||
| 2 | 3.08 | |||||||||
| 3 | 4.63 | |||||||||
| 4 | ||||||||||
| 5 | 4.26 | |||||||||
| 6 | 4.49 | |||||||||
| 7 | 4.27 | |||||||||
| 8 | ||||||||||
| 9 | 2.31 | 9.78 | 2.20 | 2.64 | 5.05 | 1.96 | 1.20 | 1.58 | 1.75 | 2.64 |
| 10 | ||||||||||
| 11 | ||||||||||
| 12 | 4.21 | |||||||||
| 13 | 4.14 | |||||||||
| 14 | ||||||||||
| 15 | 3.80 | |||||||||
| 16 | 2.53 | 1.05 | 2.12 | 1.83 | 5.07 | 2.64 | 1.20 | 1.62 | 1.73 | 2.72 |
| 17 | 2.31 | 9.78 | 2.20 | 2.64 | 5.05 | 1.96 | 1.20 | 1.58 | 1.75 | 2.64 |
| 18 | 2.87 | 1.15 | 8.24 | 1.31 | 3.03 | 6.28 | 5.27 | 8.54 | 7.53 | 1.04 |
| 19 | 4.37 | |||||||||
| 20 | 3.04 | |||||||||
| 21 | 1.15 | 4.74 | 5.10 | 5.99 | 1.99 | 7.36 | 4.48 | 6.56 | 7.70 | 1.11 |
| 22 | 1.00 | 4.20 | 2.52 | 3.20 | 9.42 | 3.91 | 2.08 | 3.25 | 4.14 | 6.59 |
| 23 | ||||||||||
| 24 | ||||||||||
Appendix A.2. Correlation Matrix
| F | Rb | Na | Cl | SWI | Li | SO | K | DOC | Sr | B | Sr/Sr | Br | Mg | HCO | EC | Ca | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 1 | ||||||||||||||||
| Rb | 0.27 | 1 | |||||||||||||||
| Na | 0.20 | 0.43 | 1 | ||||||||||||||
| Cl | 0.40 | 0.48 | 0.83 | 1 | |||||||||||||
| SWI | 0.48 | 0.41 | 0.49 | 0.48 | 1 | ||||||||||||
| Li | 0.48 | 0.38 | 0.64 | 0.69 | 0.62 | 1 | |||||||||||
| SO | 0.59 | 0.51 | 0.62 | 0.75 | 0.68 | 0.90 | 1 | ||||||||||
| K | 0.36 | 0.70 | 0.75 | 0.74 | 0.68 | 0.66 | 0.74 | 1 | |||||||||
| DOC | 0.33 | 0.60 | 0.50 | 0.67 | 0.59 | 0.69 | 0.78 | 0.76 | 1 | ||||||||
| Sr | 0.14 | 0.15 | 0.35 | 0.32 | 0.39 | 0.68 | 0.57 | 0.43 | 0.55 | 1 | |||||||
| B | 0.15 | 0.11 | 0.49 | 0.55 | 0.37 | 0.59 | 0.51 | 0.49 | 0.54 | 0.56 | 1 | ||||||
| Sr/Sr | −0.21 | −0.43 | −0.02 | 0.09 | 0.05 | 0.08 | 0.01 | −0.17 | 0.02 | 0.11 | 0.29 | 1 | |||||
| Br | 0.11 | 0.03 | 0.41 | 0.49 | 0.46 | 0.35 | 0.41 | 0.23 | 0.37 | 0.32 | 0.32 | 0.33 | 1 | ||||
| Mg | −0.05 | −0.53 | 0.18 | 0.11 | −0.07 | 0.13 | −0.01 | −0.28 | −0.29 | 0.18 | 0.15 | 0.51 | 0.44 | 1 | |||
| HCO | −0.14 | −0.45 | 0.32 | 0.16 | −0.06 | 0.18 | 0.05 | −0.15 | −0.23 | 0.29 | 0.21 | 0.45 | 0.52 | 0.93 | 1 | ||
| EC | 0.20 | 0.00 | 0.67 | 0.62 | 0.37 | 0.61 | 0.53 | 0.32 | 0.27 | 0.46 | 0.41 | 0.24 | 0.71 | 0.69 | 0.75 | 1 | |
| Ca | 0.06 | −0.20 | 0.49 | 0.43 | 0.18 | 0.40 | 0.32 | 0.12 | 0.08 | 0.49 | 0.36 | 0.32 | 0.62 | 0.80 | 0.86 | 0.91 | 1 |
Appendix A.3. Optimal Cluster Number for HCA

Appendix A.4. Exploratory Factor Analysis (EFA)
Appendix A.4.1. Determination of the Factor Number

Appendix A.4.2. Factor Loadings and Pre-Assessed Tests for Statistical Basic Requirements
| FA1 | FA2 | FA3 | h | u | MSA | C- | p (SWT) | |
|---|---|---|---|---|---|---|---|---|
| HCO | 0.97 | 0.94 | 0.06 | 0.5 | 0.90 | 4.9 | ||
| Mg | 0.96 | 0.92 | 0.08 | 0.7 | 0.91 | 1.3 | ||
| Ca | 0.87 | 0.88 | 0.12 | 0.7 | 0.90 | 3.4 | ||
| EC | 0.75 | 0.91 | 0.09 | 0.7 | 0.89 | 7.0 | ||
| Br | 0.51 | 0.45 | 0.55 | 0.5 | 0.90 | 6.1 | ||
| Sr/Sr | 0.47 | 0.26 | 0.74 | 0.6 | 0.91 | 1.1 | ||
| SO | 0.92 | 0.94 | 0.06 | 0.9 | 0.89 | 5.0 | ||
| Li | 0.86 | 0.86 | 0.14 | 0.7 | 0.89 | 1.4 | ||
| F | 0.71 | 0.36 | 0.64 | 0.7 | 0.90 | 1.7 | ||
| DOC | 0.71 | 0.75 | 0.25 | 0.8 | 0.90 | 1.6 | ||
| SWI-dist | 0.60 | 0.52 | 0.48 | 0.5 | 0.90 | 2.2 | ||
| B | 0.42 | 0.36 | 0.64 | 0.9 | 0.90 | 3.0 | ||
| Sr | 0.70 | 0.47 | 0.53 | 0.7 | 0.90 | 1.9 | ||
| Na | 0.93 | 0.92 | 0.08 | 0.7 | 0.89 | 2.5 | ||
| K | 0.66 | 0.86 | 0.14 | 0.8 | 0.89 | 5.3 | ||
| Cl | 0.64 | 0.80 | 0.20 | 0.7 | 0.89 | 2.7 | ||
| Rb | −0.52 | 0.52 | 0.65 | 0.35 | 0.9 | 0.91 | 1.1 | |
| Loadings | 4.33 | 4.61 | 2.91 | |||||
| Proportion Variance | 0.25 | 0.27 | 0.17 | |||||
| Cumulative Variance | 0.25 | 0.53 | 0.70 | |||||
| Overall KMO and C- | 0.7 | 0.90 |
References
- Goldscheider, N.; Mádl-Szőnyi, J.; Erőss, A.; Schill, E. Review: Thermal water resources in carbonate rock aquifers. Hydrogeol. J. 2010, 18, 1303–1318. [Google Scholar] [CrossRef] [Green Version]
- Stober, I. Hydrochemical properties of deep carbonate aquifers in the SW German Molasse basin. Geotherm. Energy 2014, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Bohnsack, D.; Potten, M.; Pfrang, D.; Wolpert, P.; Zosseder, K. Porosity–permeability relationship derived from Upper Jurassic carbonate rock cores to assess the regional hydraulic matrix properties of the Malm reservoir in the South German Molasse Basin. Geotherm. Energy 2020, 8, 12. [Google Scholar] [CrossRef]
- Konrad, F.; Savvatis, A.; Wellmann, F.; Zosseder, K. Hydraulic behavior of fault zones in pump tests of geothermal wells: A parametric analysis using numerical simulations for the Upper Jurassic aquifer of the North Alpine Foreland Basin. Geotherm. Energy 2019, 7, 25. [Google Scholar] [CrossRef]
- Barbieri, M.; Boschetti, T.; Petitta, M.; Tallini, M. Stable isotope (2H, 18O and 87Sr/86Sr) and hydrochemistry monitoring for groundwater hydrodynamics analysis in a karst aquifer (Gran Sasso, Central Italy). Appl. Geochem. 2005, 20, 2063–2081. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Z.; Su, Y.; Ma, J.; Zhang, Y. Hydrogeochemical and isotope evidence of groundwater evolution and recharge in Minqin Basin, Northwest China. J. Hydrol. 2007, 333, 239–251. [Google Scholar] [CrossRef]
- Bouchaou, L.; Michelot, J.; Vengosh, A.; Hsissou, Y.; Qurtobi, M.; Gaye, C.; Bullen, T.; Zuppi, G. Application of multiple isotopic and geochemical tracers for investigation of recharge, salinization, and residence time of water in the Souss–Massa aquifer, southwest of Morocco. J. Hydrol. 2008, 352, 267–287. [Google Scholar] [CrossRef]
- Varsányi, I.; Palcsu, L.; Kovács, L.Ó. Groundwater flow system as an archive of palaeotemperature: Noble gas, radiocarbon, stable isotope and geochemical study in the Pannonian Basin, Hungary. Appl. Geochem. 2011, 26, 91–104. [Google Scholar] [CrossRef]
- Ettayfi, N.; Bouchaou, L.; Michelot, J.; Tagma, T.; Warner, N.; Boutaleb, S.; Massault, M.; Lgourna, Z.; Vengosh, A. Geochemical and isotopic (oxygen, hydrogen, carbon, strontium) constraints for the origin, salinity, and residence time of groundwater from a carbonate aquifer in the Western Anti-Atlas Mountains, Morocco. J. Hydrol. 2012, 438–439, 97–111. [Google Scholar] [CrossRef]
- Mayer, A.; Sültenfuß, J.; Travi, Y.; Rebeix, R.; Purtschert, R.; Claude, C.; Le Gal La Salle, C.; Miche, H.; Conchetto, E. A multi-tracer study of groundwater origin and transit-time in the aquifers of the Venice region (Italy). Appl. Geochem. 2014, 50, 177–198. [Google Scholar] [CrossRef]
- Batlle-Aguilar, J.; Banks, E.W.; Batelaan, O.; Kipfer, R.; Brennwald, M.S.; Cook, P.G. Groundwater residence time and aquifer recharge in multilayered, semi-confined and faulted aquifer systems using environmental tracers. J. Hydrol. 2017, 546, 150–165. [Google Scholar] [CrossRef]
- Barbieri, M. Isotopes in Hydrology and Hydrogeology. Water 2019, 11, 291. [Google Scholar] [CrossRef] [Green Version]
- McKay, J.; Lenczewski, M.; Leal-Bautista, R.M. Characterization of Flowpath Using Geochemistry and 87Sr/86Sr Isotope Ratios in the Yalahau Region, Yucatan Peninsula, Mexico. Water 2020, 12, 2587. [Google Scholar] [CrossRef]
- Wu, C.; Wu, X.; Mu, W.; Zhu, G. Using Isotopes (H, O, and Sr) and Major Ions to Identify Hydrogeochemical Characteristics of Groundwater in the Hongjiannao Lake Basin, Northwest China. Water 2020, 12, 1467. [Google Scholar] [CrossRef]
- Castro, M.C.; Jambon, A.; de Marsily, G.; Schlosser, P. Noble gases as natural tracers of water circulation in the Paris Basin: 1. Measurements and discussion of their origin and mechanisms of vertical transport in the basin. Water Resour. Res. 1998, 34, 2443–2466. [Google Scholar] [CrossRef]
- Castro, M.C.; Goblet, P.; Ledoux, E.; Violette, S.; de Marsily, G. Noble gases as natural tracers of water circulation in the Paris Basin: 2. Calibration of a groundwater flow model using noble gas isotope data. Water Resour. Res. 1998, 34, 2467–2483. [Google Scholar] [CrossRef]
- Millot, R.; Guerrot, C.; Innocent, C.; Négrel, P.; Sanjuan, B. Chemical, multi-isotopic (Li–B–Sr–U–H–O) and thermal characterization of Triassic formation waters from the Paris Basin. Chem. Geol. 2011, 283, 226–241. [Google Scholar] [CrossRef] [Green Version]
- Szocs, T.; Rman, N.; Süveges, M.; Palcsu, L.; Tóth, G.; Lapanje, A. The application of isotope and chemical analyses in managing transboundary groundwater resources. Appl. Geochem. 2013, 32, 95–107. [Google Scholar] [CrossRef]
- Gerber, C.; Vaikmäe, R.; Aeschbach, W.; Babre, A.; Jiang, W.; Leuenberger, M.; Lu, Z.T.; Mokrik, R.; Müller, P.; Raidla, V.; et al. Using 81Kr and noble gases to characterize and date groundwater and brines in the Baltic Artesian Basin on the one-million-year timescale. Geochim. Cosmochim. Acta 2017, 205, 187–210. [Google Scholar] [CrossRef] [Green Version]
- Panda, U.C.; Sundaray, S.K.; Rath, P.; Nayak, B.B.; Bhatta, D. Application of factor and cluster analysis for characterization of river and estuarine water systems—A case study: Mahanadi River (India). J. Hydrol. 2006, 331, 434–445. [Google Scholar] [CrossRef]
- Cloutier, V.; Lefebvre, R.; Therrien, R.; Savard, M.M. Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. J. Hydrol. 2008, 353, 294–313. [Google Scholar] [CrossRef]
- Menció, A.; Folch, A.; Mas-Pla, J. Identifying key parameters to differentiate groundwater flow systems using multifactorial analysis. J. Hydrol. 2012, 472–473, 301–313. [Google Scholar] [CrossRef]
- Matiatos, I.; Alexopoulos, A.; Godelitsas, A. Multivariate statistical analysis of the hydrogeochemical and isotopic composition of the groundwater resources in northeastern Peloponnesus (Greece). Sci. Total Environ. 2014, 476–477, 577–590. [Google Scholar] [CrossRef]
- Lemcke, K.; Tunn, W. Tiefenwasser in der süddeutschen Molasse und ihrer verkarsteten Malmunterlage. Bull. Ver. Schweiz Pet. Geol. Ingenieure 1956, 23, 35–56. [Google Scholar]
- Lemcke, K. Übertiefe Grundwässer im süddeutschen Alpenvorland. Bull. Ver. Schweiz Pet. Geol. Ingenieure 1976, 42, 9–18. [Google Scholar]
- Udluft, P. Das tiefere Grundwasser zwischen Vindelicischem Rücken und Alpenrand. [The deeper groundwater between the Vindelician ridge and the margin of the Alps]. Geol. Jahrb. 1975, C11, 3–29. [Google Scholar]
- Villinger, E. Über Potentialverteilung und Strömungssysteme im Karstwasser der Schwäbischen Alb (Oberer Jura, SW-Deutschland). [On Potential Distribution and Flow Systems of the Karstwater of the Swabian Alb (Upper Jurassic, South-West-Germany)]. Geol. Jahrb. 1977, C18, 3–93. [Google Scholar]
- Andres, G.; Frisch, H. Hydrogeologie und Hydraulik im Malmkarst des Molassebeckens und der angrenzenden Fränkisch-Schwäbischen Alb. In Die Thermal- und Schwefelwasservorkommen von Bad Gögging; Andres, G., Wirth, H., Eds.; Schriftenreihe Bayerisches Landesamt für Wasserwirtschaft: München, Germany, 1981; Volume 15, Chapter 11; pp. 108–117. [Google Scholar]
- Stichler, W.; Rauert, W.; Weise, S.; Wolf, M.; Koschel, G.; Stier, P.; Prestel, R.; Hedin, K.; Bertleff, B. Isotopenhydrologische und hydrochemische Untersuchungen zur Erkundung des Fließsystems im Malmkarstaquifer des süddeutschen Alpenvorlandes. [Isotope-hydrological and Hydrochemical Investigations of the Flow System of the Malmkarst Aquifer in the Pre-Alpine Region of South Germany]. Zeitschrift der Deutschen Geologischen Gesellschaft 1987, 138, 387–398. [Google Scholar]
- Villinger, E. Bemerkungen zur Verkarstung des Malms unter dem westlichen süddeutschen Molassebecken. [Comments on the karstification of the Malm under the western molasse basin in South Germany]. Bull. Ver. Schweiz Pet. Geol. Ingenieure 1988, 54, 41–59. [Google Scholar] [CrossRef]
- Balderer, W. Hydrogeologische Charakterisierung der Grundwasservorkommen innerhalb der Molasse der Nordostschweiz aufgrund von hydrochemischen und Isotopenuntersuchungen. [Hydrogeological Characterization of the Groundwater Reservoirs in the Molasse of the North-East of Switzerland on the Basis of Hydrochemical Investigations and Isotope Methods]. Steirische BeiträGe Hydrogeol. 1990, 41, 35–104. [Google Scholar]
- Bertleff, B.; Ellwanger, D.; Szenkler, C.; Eichinger, L.; Trimborn, P.; Wolfendale, N. Interpretation of hydrochemical and hydroisotopical measurements on palaeogroundwaters in Oberschwaben, South German Alpine foreland, with focus on quaternary geology. In International Symposium on Applications of Isotope Techniques in Studying Past and Current Environmental Changes in the Hydrosphere and the Atmosphere; International Atomic Energy Agency (IAEA): Vienna, Austria, 1993; Volume IAEA-SM-329/66, pp. 337–357. [Google Scholar]
- Stichler, W. Isotopengehalte in Tiefengrundwässern aus Erdöl- und Erdgasbohrungen im süddeutschen Molassebecken. [Isotope contents of deep groundwater from oil and gas wells located in the South German Molasse basin]. Beiträge Hydrogeol. 1997, 48, 81–88. [Google Scholar]
- Prestel, R. Hydrochemische Untersuchungen im süddeutschen Molassebecken. In Schlussbericht Forschungsvorhaben 03 E 6240 A/B: Hydrogeothermische Energiebilanz und Grundwasserhaushalt des Malmkarstes im süddeutschen Molassebecken, 1991; Frisch, H., Werner, J., Eds.; Bayerisches Landesamt für Wasserwirtschaft, Geologisches Landesamt Baden-Württemberg: München, Freiburg i. Br., Germany, 1988; Chapter 4; p. 64. [Google Scholar]
- Weise, S.; Wolf, M.; Fritz, P.; Rauert, W.; Stichler, W.; Prestel, R.; Bertleff, B.; Stute, M. Isotopenhydrologische Untersuchungen im Süddeutschen Molassebecken. In Schlussbericht Forschungsvorhaben 03 E 6240 A/B: Hydrogeothermische Energiebilanz und Grundwasserhaushalt des Malmkarstes im süddeutschen Molassebecken, 1991; Frisch, H., Werner, J., Eds.; Bayerisches Landesamt für Wasserwirtschaft, Geologisches Landesamt Baden-Württemberg: München, Freiburg i. Br., Germany, 1991; Chapter 5; p. 106. [Google Scholar]
- Frisch, H.; Huber, B. Ein hydrogeologisches Modell und der Versuch einer Bilanzierung des Thermalwasservorkommens im Malmkarst des süddeutschen Molassebeckens. [A Hydrogeological Model and the Attempt of a Balance of the Thermal Water for the Malmkarst in the Southgerman and the Neighbouring Upper Austrian Molasse Basin]. Hydrogeol. Umw. 2000, 20, 25–43. [Google Scholar]
- Birner, J.; Mayr, C.; Thomas, L.; Schneider, M.; Baumann, T.; Winkler, A. Hydrochemie und Genese der tiefen Grundwässer des Malmaquifers im bayerischen Teil des süddeutschen Molassebeckens. [Hydrochemistry and evolution of deep groundwaters in the Malm aquifer in the bavarian part of the South German Molasse Basin]. Z. Geol. Wiss. 2011, 39, 291–308. [Google Scholar]
- Mayrhofer, C.; Niessner, R.; Baumann, T. Hydrochemistry and hydrogen sulfide generating processes in the Malm aquifer, Bavarian Molasse Basin, Germany. Hydrogeol. J. 2014, 22, 151–162. [Google Scholar] [CrossRef]
- Savvatis, A.; Steiner, U.; Huber, B.; Fritzer, T.; Schneider, M. Limitierungen bei der Ermittlung der Grundwasserfließrichtung in tiefen Aquiferen am Beispiel des Malms im Süddeutschen Molassebecken. [Limitations in the identification of the groundwater flow direction in deep aquifers using the example of the Malm in the Southern German Molasse Basin]. Grundwasser 2015, 20, 271–280. [Google Scholar] [CrossRef]
- Mraz, E.; Wolfgramm, M.; Moeck, I.; Thuro, K. Detailed Fluid Inclusion and Stable Isotope Analysis on Deep Carbonates from the North Alpine Foreland Basin to Constrain Paleofluid Evolution. Geofluids 2019, 2019, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Birner, J.; Fritzer, T.; Jodocy, M.; Savvatis, A.; Schneider, M.; Stober, I. Hydraulische Eigenschaften des Malmaquifers im Süddeutschen Molassebecken und ihre Bedeutung für die geothermische Erschließung. [Hydraulic characterisation of the Malm aquifer in the South German Molasse basin and its impact on geothermal exploitations]. Z. Geol. Wiss. 2012, 40, 133–156. [Google Scholar]
- Wanner, C.; Eichinger, F.; Jahrfeld, T.; Diamond, L.W. Unraveling the Formation of Large Amounts of Calcite Scaling in Geothermal Wells in the Bavarian Molasse Basin: A Reactive Transport Modeling Approach. Procedia Earth Planet. Sci. 2017, 17, 344–347. [Google Scholar] [CrossRef] [Green Version]
- Köhl, B.; Grundy, J.; Baumann, T. Rippled scales in a geothermal facility in the Bavarian Molasse Basin: A key to understand the calcite scaling process. Geotherm. Energy 2020, 8, 23. [Google Scholar] [CrossRef]
- Heidinger, M.; Eichinger, F.; Purtschert, R.; Mueller, P.; Zappala, J.; Wirsing, G.; Geyer, T.; Fritzer, T.; Groß, D. Altersbestimmung an thermalen Tiefenwässern im Oberjura des Molassebeckens mittels Krypton-Isotopen. [81Kr/85Kr-Dating of thermal groundwaters in the Upper Jurassic (Molasse Basin)]. Grundwasser 2019, 24, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, G.; Müller, M.; Weggen, K. Evolution of the Molasse Basin (Germany, Switzerland). Tectonophysics 1987, 137, 77–92. [Google Scholar] [CrossRef]
- Freudenberger, W.; Schwerd, K. Erläuterungen zur Geologischen Karte von Bayern 1:500000, 4th ed.; Bayerisches Geologisches Landesamt: Munich, Germany, 1996; p. 329. [Google Scholar]
- Doppler, G.; Heissig, K.; Reichenbacher, B. Die Gliederung des Tertiars im sueddeutschen Molassebecken. Newsl. Stratigr. 2006, 41, 359–375. [Google Scholar] [CrossRef]
- Lemcke, K. Geologie von Bayern I.-Das bayerische Alpenvorland vor der Eiszeit-Erdgeschichte-Bau-Bodenschätze; Schweizerbart: Stuttgart, Germany, 1988; p. 175. [Google Scholar]
- Drews, M.C.; Seithel, R.; Savvatis, A.; Kohl, T.; Stollhofen, H. A normal-faulting stress regime in the Bavarian Foreland Molasse Basin? New evidence from detailed analysis of leak-off and formation integrity tests in the greater Munich area, SE-Germany. Tectonophysics 2019, 755, 1–9. [Google Scholar] [CrossRef]
- Agemar, T.; Alten, J.A.; Ganz, B.; Kuder, J.; Kühne, K.; Schumacher, S.; Schulz, R. The Geothermal Information System for Germany—GeotIS. Z. der Dtsch. Ges. für Geowiss. 2014, 165, 129–144. [Google Scholar] [CrossRef]
- Agemar, T.; Weber, J.; Schulz, R. Deep Geothermal Energy Production in Germany. Energies 2014, 7, 4397–4416. [Google Scholar] [CrossRef]
- Böhm, F.; Savvatis, A.; Steiner, U.; Schneider, M.; Koch, R. Lithofazielle Reservoircharakterisierung zur geothermischen Nutzung des Malm im Großraum München. [Lithofacies and characterization of the geothermal Malm reservoir in the greater area of Munich]. Grundwasser 2013, 18, 3–13. [Google Scholar] [CrossRef]
- Konrad, F.; Savvatis, A.; Degen, D.; Wellmann, F.; Einsiedl, F.; Zosseder, K. Productivity enhancement of geothermal wells through fault zones: Efficient numerical evaluation of a parameter space for the Upper Jurassic aquifer of the North Alpine Foreland Basin. Geothermics 2021. (accepted). [Google Scholar]
- Furtak, H.; Langguth, H. Zur hydrochemischen Kennzeichnung von Grundwässern und Grundwassertypen mittels Kennzahlen. Mem. IAH-Congr. 1967, VII, 86–96. [Google Scholar]
- Hahn-Weihnheimer, P.; Hirner, A.; Lemcke, K. Zur Herkunft süddeutscher Erdöle—Geochemische Ergebnisse und Versuch einer geologischen Interpretation. [On the origin of crude oils from Southern Germany—Geochemical results and a tentative geological interpretation]. Bull. Der Ver. Schweiz Pet. Geol. Ingenieure 1979, 45, 35–46. [Google Scholar] [CrossRef]
- Stober, I.; Wolfgramm, M.; Birner, J. Hydrochemie der Tiefenwässer in Deutschland. [Hydrochemistry of deep waters in Germany]. Z. Geol. Wiss. 2014, 41/42, 339–380. [Google Scholar]
- Bertleff, B.; Watzel, R. Tiefe Aquifersysteme im südwestdeutschen Molassebecken. Eine umfassende hydrogeologische Analyse als Grundlage eines zukünftigen Quantitäts- und Qualitätsmanagements. [Deep Aquifer Systems in the Southwest German Molasse basin. An extensive hydro-geological analysis as the basis for future quantitative and qualitative groundwater management]. Abh. Geol. Landesamt Baden Württemberg 2002, 15, 75–90. [Google Scholar]
- Heine, F.; Einsiedl, F. Groundwater dating with dissolved organic radiocarbon: A promising approach in carbonate aquifers. Appl. Geochem. 2021, 125. [Google Scholar] [CrossRef]
- Dehnert, J.; Nestler, W.; Freyer, K.; Treutler, H.; Neitzel, P.L.; Walther, W. Bestimmung der notwendigen Abpumpzeiten von Grundwasserbeobachtungsrohren mit Hilfe der natürlichen Radonaktivitätskonzentration. In Grundwasser und Rohstoffgewinnung. GeoCongress 2; Merkel, B., Dietrich, P.G., Struckmeier, W., Löhnert, E.P., Eds.; 1996; pp. 40–45. Available online: https://www.researchgate.net/publication/313730398_Bestimmung_der_notwendigen_Abpumpzeiten_von_Grundwasserbeobachtungsrohren_mit_Hilfe_der_naturlichen_Radonaktivitatskonzentration (accessed on 20 April 2021).
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- Kharaka, Y.; Hanor, J. Deep Fluids in Sedimentary Basins. In Treatise on Geochemistry; Holland, H., Turekian, K., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2014; Volume 7, pp. 471–515. [Google Scholar] [CrossRef]
- Stumpp, C.; Klaus, J.; Stichler, W. Analysis of long-term stable isotopic composition in German precipitation. J. Hydrol. 2014, 517, 351–361. [Google Scholar] [CrossRef]
- Rosner, M. Geochemical and instrumental fundamentals for accurate and precise strontium isotope data of food samples: Comment on “Determination of the strontium isotope ratio by ICP-MS ginseng as a tracer of regional origin” (Choi et al., 2008). Food Chem. 2010, 121, 918–921. [Google Scholar] [CrossRef]
- Sültenfuß, J.; Roether, W.; Rhein, M. The Bremen mass spectrometric facility for the measurement of helium isotopes, neon, and tritium in water. Isot. Environ. Health Stud. 2009, 45, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Aeschbach-Hertig, W.; Solomon, D.K. Noble Gas Thermometry in Groundwater Hydrology. In The Noble Gases as Geochemical Tracers; Burnard, P., Ed.; Advances in Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 2013; pp. 81–122. [Google Scholar] [CrossRef]
- Andrews, J.N.; Lee, D.J. Inert gases in groundwater from the Bunter Sandstone of England as indicators of age and palaeoclimatic trends. J. Hydrol. 1979, 41, 233–252. [Google Scholar] [CrossRef]
- Andrews, J.; Goldbrunner, J.; Darling, W.; Hooker, P.; Wilson, G.; Youngman, M.; Eichinger, L.; Rauert, W.; Stichler, W. A radiochemical, hydrochemical and dissolved gas study of groundwaters in the Molasse basin of Upper Austria. Earth Planet. Sci. Lett. 1985, 73, 317–332. [Google Scholar] [CrossRef]
- Aeschbach-Hertig, W.; Peeters, F.; Beyerle, U.; Kipfer, R. Palaeotemperature reconstruction from noble gases in ground water taking into account equilibration with entrapped air. Nature 2000, 405, 1040–1044. [Google Scholar] [CrossRef] [Green Version]
- Kipfer, R.; Aeschbach-Hertig, W.; Peeters, F.; Stute, M. Noble Gases in Lakes and Ground Waters. In Noble Gases; Porcelli, D.P., Ballentine, C.J., Wieler, R., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2002; Volume 47, Chapter 14; pp. 615–700. [Google Scholar] [CrossRef] [Green Version]
- Stute, M.; Schlosser, P. Principles and Applications of the Noble Gas Paleothermometer. In Climate Change in Continental Isotopic Records; Swart, P., Lohmann, K.C., McKenzie, J., Savin, S., Eds.; AGU: Washington, DC, USA, 1993; pp. 89–100. [Google Scholar] [CrossRef]
- Wilson, G.; McNeill, G. Noble gas recharge temperatures and the excess air component. Appl. Geochem. 1997, 12, 747–762. [Google Scholar] [CrossRef]
- Jung, M.; Aeschbach, W. A new software tool for the analysis of noble gas data sets from (ground)water. Environ. Model. Softw. 2018, 103, 120–130. [Google Scholar] [CrossRef]
- Jung, M.; Wieser, M.; von Oehsen, A.; Aeschbach-Hertig, W. Properties of the closed-system equilibration model for dissolved noble gases in groundwater. Chem. Geol. 2013, 339, 291–300. [Google Scholar] [CrossRef]
- Kulongoski, J.; Hilton, D. Applications of Groundwater Helium. In Handbook of Environmental Isotope Geochemistry; Advances in Isotope Geochemistry; Baskaran, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Chapter 15; pp. 285–304. [Google Scholar] [CrossRef]
- Bethke, C.M.; Johnson, T.M. Paradox of groundwater age. Geology 2002, 30, 107–110. [Google Scholar] [CrossRef]
- Tolstikhin, I.; Lehmann, B.; Loosli, H.; Gautschi, A. Helium and argon isotopes in rocks, minerals, and related ground waters: A case study in northern Switzerland. Geochim. Cosmochim. Acta 1996, 60, 1497–1514. [Google Scholar] [CrossRef]
- Kulongoski, J.T.; Hilton, D.R.; Izbicki, J.A. Helium isotope studies in the Mojave Desert, California: Implications for groundwater chronology and regional seismicity. Chem. Geol. 2003, 202, 95–113. [Google Scholar] [CrossRef]
- Clarke, W.; Jenkins, W.; Top, Z. Determination of tritium by mass spectrometric measurement of 3He. Int. J. Appl. Radiat. Isot. 1976, 27, 515–522. [Google Scholar] [CrossRef]
- Ballentine, C.J.; Burnard, P.G. Production, Release and Transport of Noble Gases in the Continental Crust. Rev. Mineral. Geochem. 2002, 47, 481–538. [Google Scholar] [CrossRef]
- Phillips, F.; Castro, M. Groundwater Dating and Residence-Time Measurements. In Treatise on Geochemistry; Holland, H., Turekian, K., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2014; Volume 7, pp. 361–400. [Google Scholar] [CrossRef]
- Graham, D.W. Noble Gas Isotope Geochemistry of Mid-Ocean Ridge and Ocean Island Basalts: Characterization of Mantle Source Reservoirs. Rev. Mineral. Geochem. 2002, 47, 247–317. [Google Scholar] [CrossRef]
- Lippmann, J.; Stute, M.; Torgersen, T.; Moser, D.; Hall, J.; Lin, L.; Borcsik, M.; Bellamy, R.; Onstott, T. Dating ultra-deep mine waters with noble gases and 36Cl, Witwatersrand Basin, South Africa. Geochim. Cosmochim. Acta 2003, 67, 4597–4619. [Google Scholar] [CrossRef]
- Ozima, M.; Podosek, F.A. Noble Gas Geochemistry; Cambridge University Press: Cambridge, UK, 2002; p. 286. [Google Scholar]
- Lee, J.Y.; Marti, K.; Severinghaus, J.P.; Kawamura, K.; Yoo, H.S.; Lee, J.B.; Kim, J.S. A redetermination of the isotopic abundances of atmospheric Ar. Geochim. Cosmochim. Acta 2006, 70, 4507–4512. [Google Scholar] [CrossRef]
- Andrews, J.N.; Youngman, M.J.; Goldbrunner, J.E.; Darling, W.G. The geochemistry of formation waters in the molasse basin of upper Austria. Environ. Geol. Water Sci. 1987, 10, 43–57. [Google Scholar] [CrossRef]
- Weise, S.; Moser, H. Groundwater dating with helium isotopes. In International Symposium on the Use of Isotope Techniques in Water Resources Development, 1987; International Atomic Energy Agency (IAEA): Vienna, Austria, 1987; Volume IAEA-SM-299/44, pp. 105–126. [Google Scholar]
- Osenbrück, K.; Lippmann, J.; Sonntag, C. Dating very old pore waters in impermeable rocks by noble gas isotopes. Geochim. Cosmochim. Acta 1998, 62, 3041–3045. [Google Scholar] [CrossRef]
- Torgersen, T.; Stute, M. Helium (and other noble gases) as a tool for understanding long time-scale groundwater transport. In Isotope Methods for Dating Old Groundwater; International Atomic Energy Agency (IAEA): Vienna, Austria, 2013; Chapter 8; pp. 179–244. [Google Scholar]
- Petersen, J.; Deschamps, P.; Hamelin, B.; Fourré, E.; Gonçalvès, J.; Zouari, K.; Guendouz, A.; Michelot, J.L.; Massault, M.; Dapoigny, A.; et al. Groundwater flowpaths and residence times inferred by 14C, 36Cl and 4He isotopes in the Continental Intercalaire aquifer (North-Western Africa). J. Hydrol. 2018, 560, 11–23. [Google Scholar] [CrossRef]
- Weiss, R.F. Solubility of helium and neon in water and seawater. J. Chem. Eng. Data 1971, 16, 235–241. [Google Scholar] [CrossRef]
- Stute, M.; Sonntag, C.; Deák, J.; Schlosser, P. Helium in deep circulating groundwater in the Great Hungarian Plain: Flow dynamics and crustal and mantle helium fluxes. Geochim. Cosmochim. Acta 1992, 56, 2051–2067. [Google Scholar] [CrossRef]
- Torgersen, T.; Clarke, W.B. Helium accumulation in groundwater, I: An evaluation of sources and the continental flux of crustal 4He in the Great Artesian Basin, Australia. Geochim. Cosmochim. Acta 1985, 49, 1211–1218. [Google Scholar] [CrossRef]
- Thuro, K.; Zosseder, K.; Bohnsack, D.; Heine, F.; Konrad, F.; Mraz, E.; Stockinger, G. Dolomitkluft: Erschließung, Test und Analyse des ersten kluftdominierten Dolomitaquifers im tiefen Malm des Molassebeckens zur Erhöhung der Erfolgsaussichten; Technical Report; Technical University of Munich: Munich, Germany, 2019. [Google Scholar]
- Belkhiri, L.; Boudoukha, A.; Mouni, L.; Baouz, T. Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater—A case study: Ain Azel plain (Algeria). Geoderma 2010, 159, 390–398. [Google Scholar] [CrossRef]
- Kassambara, A. Practical Guide to Cluster Analysis in R, 1st ed.; 2017; p. 187. Available online: https://www.datanovia.com/en/product/practical-guide-to-cluster-analysis-in-r/ (accessed on 21 April 2021).
- Schumacker, R.E. Using R with Multivariate Statistics; SAGE Publications: Los Angeles, CA, USA, 2016; p. 408. [Google Scholar]
- Tabachnik, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson: New York, NY, USA, 2013; p. 983. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Revelle, W. psych: Procedures for Psychological, Psychometric, and Personality Research; Northwestern University: Evanston, IL, USA, 2019. [Google Scholar]
- Jorgensen, T.D.; Pornprasertmanit, S.; Schoemann, A.M.; Rosseel, Y. semTools: Useful Tools for Structural Equation Modeling; 2000. Available online: https://CRAN.R-project.org/package=semTools (accessed on 21 April 2021).
- Wickham, H. Elegant Graphics for Data Analysis: Ggplot2; Springer: New York, NY, USA, 2008; pp. 21–54. [Google Scholar] [CrossRef]
- Backhaus, K.; Erichson, B.; Plinke, W.; Weiber, R. Multivariate Analysemethoden; Springer: Berlin/Heidelberg, Germany, 2016; Volume 14, p. 854. [Google Scholar] [CrossRef]
- DiStefano, C.; Zhu, M.; Mîndrilă, D. Understanding and Using Factor Scores: Considerations for the Applied Researcher. Pract. Assess. Res. Eval. 2009, 14, 1–11. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Charrad, M.; Ghazzali, N.; Boiteau, V.; Niknafs, A. NbClust: An R Package for Determining the Relevant Number of Clusters in a Data Set. J. Stat. Softw. 2014, 61. [Google Scholar] [CrossRef] [Green Version]
- Horn, J.L. A rationale and test for the number of factors in factor analysis. Psychometrika 1965, 30, 179–185. [Google Scholar] [CrossRef]
- Kaiser, H.F. The Application of Electronic Computers to Factor Analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Braeken, J.; van Assen, M.A.L.M. An empirical Kaiser criterion. Psychol. Methods 2017, 22, 450–466. [Google Scholar] [CrossRef] [Green Version]
- Cattell, R.B. The Scree Test for the Number of Factors. Multivar. Behav. Res. 1966, 1, 245–276. [Google Scholar] [CrossRef]
- Zwick, W.R.; Velicer, W.F. Factors Influencing Four Rules for Determining the Number of Components to Retain. Psychol. Bull. 1986, 99, 432–442. [Google Scholar] [CrossRef]
- Costello, A.B.; Osborne, J.W. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Pract. Assess. Res. Eval. 2005, 10, 9. [Google Scholar]
- Faure, G.; Powell, J.L. Strontium Isotope Geology. In Minerals, Rocks and Inorganic Materials; Von Engelhardt, W., Hahn, T., Roy, R., Wyllie, P., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1972; Chapter 5; p. 188. [Google Scholar] [CrossRef]
- Whittemore, D.O. Geochemical differentiation of oil and gas brine from other saltwalter sources contaminating water resources: Case studies from Kansas and Oklahoma. Environ. Geosci. 1995, 2, 15–31. [Google Scholar]
- Collins, A.G. Geochemistry of Oilfield Waters, 1st ed.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; New York, NY, USA, 1975; p. 496. [Google Scholar]
- Stober, I.; Bucher, K. Herkunft der Salinität in Tiefenwässern des Grundgebirges—Unter besonderer Berücksichtigung der Kristallinwässer des Schwarzwaldes. Grundwasser 2000, 3, 125–140. [Google Scholar] [CrossRef]
- Gemici, Ü.; Tarcan, G. Distribution of boron in thermal waters of western Anatolia, Turkey, and examples of their environmental impacts. Environ. Geol. 2002, 43, 87–98. [Google Scholar] [CrossRef]
- Clark, I. Groundwater Geochemistry and Isotopes; CRC Press Taylore & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2015; p. 421. [Google Scholar]
- Langmuir, D. The geochemistry of some carbonate ground waters in central Pennsylvania. Geochim. Cosmochim. Acta 1971, 35, 1023–1045. [Google Scholar] [CrossRef]
- Mayo, A.L.; Loucks, M.D. Solute and isotopic geochemistry and ground water flow in the central Wasatch Range, Utah. J. Hydrol. 1995, 172, 31–59. [Google Scholar] [CrossRef]
- Clayton, R.N.; Friedman, I.; Graf, D.L.; Mayeda, T.K.; Meents, W.F.; Shimp, N.F. The origin of saline formation waters: 1. Isotopic composition. J. Geophys. Res. 1966, 71, 3869–3882. [Google Scholar] [CrossRef]
- Bottomley, D.J.; Conrad Gregoire, D.; Raven, K.G. Saline ground waters and brines in the Canadian Shield: Geochemical and isotopic evidence for a residual evaporite brine component. Geochim. Cosmochim. Acta 1994, 58, 1483–1498. [Google Scholar] [CrossRef]
- Van Geldern, R.; Baier, A.; Subert, H.L.; Kowol, S.; Balk, L.; Barth, J.A. Pleistocene paleo-groundwater as a pristine fresh water resource in southern Germany—Evidence from stable and radiogenic isotopes. Sci. Total Environ. 2014, 496, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Weise, S.; Stichler, W. Edelgasisotopen-Methoden als Werkzeug zur Untersuchung tiefreichender Grundwasser-Fließsysteme am Beispiel des süddeutschen Molassebeckens. [Noble gas isotope methods as a tool for investigations of deep circulating groundwater flowsystems in the South German Molasse basin]. Beitr. Hydrogeol. 1997, 48, 69–80. [Google Scholar]
- Goldbrunner, J.E. Zum Stand der geothermischen und balneologischen Tiefengrundwassernutzung im Oststeirischen Becken und im Oberösterreichischen Molassebecken. [The Status of Geothermic and Balneologic Utilization of Deep Groundwater in the East Styrian Basin and the Upper Austrian Molasse Basin]. Z. Dtsch. Geol. Ges. 1987, 138, 513–526. [Google Scholar]
- Capo, R.C.; Stewart, B.W.; Chadwick, O.A. Strontium isotopes as tracers of ecosystem processes: Theory and methods. Geoderma 1998, 82, 197–225. [Google Scholar] [CrossRef]
- Probst, A.; El Gh’mari, A.; Aubert, D.; Fritz, B.; McNutt, R. Strontium as a tracer of weathering processes in a silicate catchment polluted by acid atmospheric inputs, Strengbach, France. Chem. Geol. 2000, 170, 203–219. [Google Scholar] [CrossRef] [Green Version]
- Shand, P.; Darbyshire, D.; Love, A.; Edmunds, W. Sr isotopes in natural waters: Applications to source characterisation and water–rock interaction in contrasting landscapes. Appl. Geochem. 2009, 24, 574–586. [Google Scholar] [CrossRef]
- Baublys, K.A.; Hamilton, S.K.; Hofmann, H.; Golding, S.D. A strontium (87Sr/86Sr) isotopic study on the chemical evolution and migration of groundwaters in a low-rank coal seam gas reservoir (Surat Basin, Australia). Appl. Geochem. 2019, 101, 1–18. [Google Scholar] [CrossRef]
- Veizer, J.; Ala, D.; Azmy, K.; Bruckschen, P.; Buhl, D.; Bruhn, F.; Carden, G.; Diener, A.; Ebneth, S.; Godderis, Y.; et al. 87Sr/86Sr, 13C and 18O evolution of Phanerozoic seawater. Chem. Geol. 1999, 161, 59–88. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, S.; Broedel, V.; Clauer, N. Strontium isotopic evolution of oil-field waters from carbonate reservoir rocks in Bindley field, central Kansas, U.S.A. Geochim. Cosmochim. Acta 1987, 51, 45–53. [Google Scholar] [CrossRef]
- Heaton, T.; Vogel, J. “Excess air” in groundwater. J. Hydrol. 1981, 50, 201–216. [Google Scholar] [CrossRef]
- Peeters, F.; Beyerle, U.; Aeschbach-Hertig, W.; Holocher, J.; Brennwald, M.S.; Kipfer, R. Improving noble gas based paleoclimate reconstruction and groundwater dating using 20Ne/22Ne ratios. Geochim. Cosmochim. Acta 2003, 67, 587–600. [Google Scholar] [CrossRef] [Green Version]
- Blaser, P.; Coetsiers, M.; Aeschbach-Hertig, W.; Kipfer, R.; Van Camp, M.; Loosli, H.; Walraevens, K. A new groundwater radiocarbon correction approach accounting for palaeoclimate conditions during recharge and hydrochemical evolution: The Ledo-Paniselian Aquifer, Belgium. Appl. Geochem. 2010, 25, 437–455. [Google Scholar] [CrossRef]
- Lippmann, J.; Erzinger, J.; Zimmer, M.; Schloemer, S.; Eichinger, L.; Faber, E. On the geochemistry of gases and noble gas isotopes (including 222Rn) in deep crustal fluids: The 4000 m KTB-pilot hole fluid production test 2002-03. Geofluids 2005, 5, 52–66. [Google Scholar] [CrossRef]
- Nakata, K.; Hasegawa, T.; Solomon, D.; Miyakawa, K.; Tomioka, Y.; Ohta, T.; Matsumoto, T.; Hama, K.; Iwatsuki, T.; Ono, M.; et al. Degassing behavior of noble gases from groundwater during groundwater sampling. Appl. Geochem. 2019, 104, 60–70. [Google Scholar] [CrossRef]
- Sano, Y.; Fischer, T.P. The Analysis and Interpretation of Noble Gases in Modern Hydrothermal Systems. In The Noble Gases as Geochemical Tracers; Burnard, P., Ed.; Advances in Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 2013; pp. 249–317. [Google Scholar] [CrossRef]
- Wasserburg, G.; Mazor, E.; Zartman, R. Isotopic and chemical composition of some terrestrial natural gases. In Earth Science and Meteorites; Geiss, J., Goldberg, E., Eds.; North-Holland Pub. Co.: Amsterdam, NL, USA, 1963; pp. 219–240. [Google Scholar]
- Torgersen, T.; Kennedy, B.; Hiyagon, H.; Chiou, K.; Reynolds, J.; Clarke, W. Argon accumulation and the crustal degassing flux of40Ar in the Great Artesian Basin, Australia. Earth Planet. Sci. Lett. 1989, 92, 43–56. [Google Scholar] [CrossRef]
- Goldbrunner, J. Hydrogeology of Deep Groundwaters in Austria. Mitteilungen Osterreichischen Geol. Ges. 1999, 92, 281–294. [Google Scholar]









| Summary of all Samples | Water Type 1a | Water Type 1b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | min | max | min | max | mean ± SD | min | max | mean ± SD | |
| pH-value (-) | 6.3 | 7.5 | 6.3 | 6.7 | 6.5 ± 0.2 | 6.4 | 6.4 | 6.4 ± 0.0 | |
| EC (µS/cm) | 587 | 7702 | 587 | 746 | 671 ± 55 | 1029 | 1087 | 1058 ± 41 | |
| Ca (mmol/L) | 0.09 | 3.37 | 0.09 | 0.67 | 0.43 ± 0.20 | 0.90 | 0.95 | 0.92 ± 0.04 | |
| Mg (mmol/L) | 0.04 | 1.19 | 0.04 | 0.16 | 0.10 ± 0.04 | 0.17 | 0.21 | 0.19 ± 0.03 | |
| Ca/Mg (-) | 1.2 | 6.2 | 1.4 | 6.2 | 4.6 ± 1.5 | 4.2 | 5.5 | 4.8 ± 0.9 | |
| Na (mmol/L) | 5.15 | 80.64 | 5.15 | 6.24 | 5.60 ± 0.36 | 7.51 | 7.57 | 7.54 ± 0.04 | |
| K (mmol/L) | 0.19 | 2.18 | 0.40 | 0.59 | 0.49 ± 0.07 | 0.80 | 0.82 | 0.81 ± 0.01 | |
| Li (mmol/L) | 0.014 | 0.275 | 0.014 | 0.027 | 0.021 ± 0.004 | 0.034 | 0.038 | 0.036 ± 0.003 | |
| Sr (mmol/L) | 0.002 | 0.073 | 0.002 | 0.011 | 0.013 ± 0.006 | 0.012 | 0.016 | 0.014 ± 0.003 | |
| Rb (µmol/l) | 0.17 | 1.76 | 0.50 | 0.78 | 0.67 ± 0.09 | 1.09 | 1.37 | 1.23 ± 0.20 | |
| HCO (mmol/L) | 3.4 | 14.0 | 3.4 | 4.7 | 4.2 ± 0.5 | 5.1 | 5.1 | 5.1 ± 0.0 | |
| Cl (mmol/L) | 1.95 | 70.10 | 1.95 | 2.70 | 2.23 ± 0.23 | 4.43 | 4.45 | 4.44 ± 0.01 | |
| SO (mmol/L) | 0.00 | 3.51 | 0.04 | 0.16 | 0.09 ± 0.05 | 0.21 | 0.32 | 0.27 ± 0.08 | |
| F (mmol/L) | 0.10 | 0.42 | 0.11 | 0.42 | 0.22 ± 0.10 | 0.24 | 0.41 | 0.32 ± 0.12 | |
| Br (mmol/L) | 0.002 | 0.124 | 0.002 | 0.007 | 0.005 ± 0.002 | 0.007 | 0.011 | 0.009 ± 0.003 | |
| Boron (mmol/L) | 0.05 | 3.24 | 0.08 | 0.21 | 0.16 ± 0.06 | 0.27 | 0.31 | 0.29 ± 0.02 | |
| DOC (mg/L) | 0.50 | 70.49 | 1.48 | 2.61 | 1.90 ± 0.40 | 3.80 | 4.20 | 4.00 ± 0.28 | |
| O (‰ VSMOW) | −12.0 | −2.6 | −11.8 | −11.0 | −11.5 ± 0.2 | −11.0 | −10.8 | −10.9 ± 0.1 | |
| D (‰ VSMOW) | −86.8 | −60.6 | −86.5 | -84.8 | −85.8 ± 0.6 | −84.3 | −82.8 | −83.5 ± 1.0 | |
| SWI-dist (-) | 0.09 | 6.23 | 0.20 | 0.82 | 0.43 ± 0.18 | 0.81 | 0.84 | 0.82 ± 0.02 | |
| Sr/Sr (-) | 0.70862 | 0.70970 | 0.70881 | 0.70925 | 0.70908 ± 0.00014 | 0.70862 | 0.70899 | 0.70881 ± 0.00026 | |
| He (ccSTP/g) | 1.68 | 1.15 | 1.68 | 2.53 | 2.27 ± 0.34 | 4.22 | 1.58 | 1.00 ± 0.82 | |
| He/He (-) | 6.26 | 1.63 | 9.07 | 1.13 | 1.03 ± 0.09 | 6.45 | 7.31 | 6.88 ± 0.61 | |
| Ar (ccSTP/g) | 9.39 | 3.02 | 4.95 | 5.05 | 5.00 ± 0.07 | 3.02 | |||
| Ar/Ar (-) | 296.2 | 392.0 | 296.2 | 301.2 | 298.7 ± 3.5 | 307.6 | |||
| Water Type 2a | Water Type 2b | Water Type 3 | |||||||
| Parameter | min | max | mean ± SD | min | max | mean ± SD | min | max | mean ± SD |
| pH-value (-) | 6.8 | 7.2 | 7.0 ± 0.1 | 7.0 | 7.5 | 7.2 ± 0.3 | 6.6 | 6.9 | 6.7 ± 0.2 |
| EC (µS/cm) | 697 | 756 | 723 ± 22 | 820 | 965 | 893 ± 103 | 1596 | 7702 | 4366 ± 3092 |
| Ca (mmol/L) | 0.68 | 0.96 | 0.81 ± 0.09 | 0.85 | 1.09 | 0.97 ± 0.17 | 0.98 | 3.37 | 2.21 ± 1.20 |
| Mg (mmol/L) | 0.30 | 0.44 | 0.38 ± 0.05 | 0.42 | 0.74 | 0.58 ± 0.22 | 0.16 | 1.19 | 0.64 ± 0.52 |
| Ca/Mg (-) | 1.9 | 2.5 | 2.1 ± 0.2 | 1.2 | 2.6 | 1.9 ± 1.0 | 2.9 | 6.0 | 4.3 ± 1.5 |
| Na (mmol/L) | 5.22 | 5.65 | 5.33 ± 0.13 | 5.98 | 6.52 | 6.25 ± 0.38 | 11.70 | 80.64 | 42.27 ± 35.13 |
| K (mmol/L) | 0.19 | 0.39 | 0.35 ± 0.06 | 0.39 | 0.41 | 0.40 ± 0.01 | 0.89 | 2.18 | 1.46 ± 0.66 |
| Li (mmol/L) | 0.014 | 0.028 | 0.018 ± 0.005 | 0.014 | 0.019 | 0.017 ± 0.003 | 0.055 | 0.275 | 0.154 ± 0.112 |
| Sr (mmol/L) | 0.006 | 0.008 | 0.007 ± 0.001 | 0.002 | 0.002 | 0.002 ± 0.000 | 0.020 | 0.073 | 0.046 ± 0.027 |
| Rb (µmol/l) | 0.25 | 0.42 | 0.35 ± 0.07 | 0.46 | 0.48 | 0.47 ± 0.02 | 0.17 | 1.76 | 0.86 ± 0.81 |
| HCO (mmol/L) | 4.9 | 5.8 | 5.4 ± 0.3 | 6.7 | 6.8 | 6.7 ± 0.0 | 5.2 | 14.0 | 9.1 ± 4.5 |
| Cl (mmol/L) | 1.96 | 2.33 | 2.04 ± 0.12 | 2.25 | 2.64 | 2.45 ± 0.28 | 7.88 | 70.10 | 35.10 ± 31.83 |
| SO (mmol/L) | 0.01 | 0.14 | 0.06 ± 0.05 | 0.00 | 0.05 | 0.03 ± 0.03 | 0.47 | 3.51 | 1.67 ± 1.61 |
| F (mmol/L) | 0.10 | 0.35 | 0.17 ± 0.09 | 0.14 | 0.12 | 0.27 | 0.21 ± 0.08 | ||
| Br (mmol/L) | 0.003 | 0.016 | 0.006 ± 0.004 | 0.005 | 0.007 | 0.006 ± 0.001 | 0.008 | 0.124 | 0.057 ± 0.060 |
| Boron (mmol/L) | 0.05 | 0.23 | 0.13 ± 0.08 | 0.06 | 0.08 | 0.07 ± 0.02 | 0.81 | 3.24 | 1.84 ± 1.26 |
| DOC (mg/L) | 0.54 | 1.67 | 0.95 ± 0.33 | 0.50 | 0.84 | 0.67 ± 0.24 | 4.43 | 70.49 | 31.99 ± 34.36 |
| O (‰ VSMOW) | −12.0 | −11.5 | −11.7 ± 0.1 | −11.7 | −11.4 | −11.6 ± 0.2 | −10.5 | −2.6 | −6.9 ± 4.0 |
| D (‰ VSMOW) | −86.8 | −85.4 | −86.2 ± 0.4 | −85.7 | −84.0 | −84.9 ± 1.2 | −82.3 | −60.6 | −72.5 ± 11.0 |
| SWI-dist (-) | 0.09 | 0.49 | 0.31 ± 0.11 | 0.26 | 0.35 | 0.30 ± 0.06 | 1.07 | 6.23 | 3.41 ± 2.61 |
| Sr/Sr (-) | 0.70907 | 0.70926 | 0.70921 ± 0.00006 | 0.70901 | 0.70926 | 0.70913 ± 0.00018 | 0.70944 | 0.70970 | 0.70955 ± 0.00013 |
| He (ccSTP/g) | 1.80 | 2.32 | 2.21 ± 0.20 | 5.44 | 1.00 | 8.98 ± 3.16 | |||
| He/He (-) | 9.89 | 1.36 | 1.12 ± 0.14 | 6.26 | 8.08 | 7.22 ± 0.91 | |||
| Ar (ccSTP/g) | 5.03 | 9.39 | 1.98 | 1.46 ± 0.74 | |||||
| Ar/Ar (-) | 302.5 | 345.1 | 392.0 | 368.6 ± 33.2 | |||||
| ID | Water Type | AMC | Ffit | Tfit | Tfit_err | TMC | TMC_err |
|---|---|---|---|---|---|---|---|
| (ccSTP/g) | (-) | (°C) | |||||
| 9 | 2a | 0.32 | 0.94 | 1.4 | 0.4 | 1.4 | 0.5 |
| 16 | 1a | 0.26 | 0.97 | 0.9 | 0.5 | 0.9 | 0.4 |
| 17 | 1a | 0.23 | 0.98 | 1.4 | 0.4 | 1.4 | 0.4 |
| 18 | 1b | 0.36 | 0.45 | 3.2 | 0.5 | 3.1 | 0.5 |
| 21 | 3 | −1.26 | - | - | - | - | - |
| 22 | 3 | −0.02 | - | - | - | - | - |
| ID | Heex | Arex | tKr | JHe | JAr | tHe | tAr |
|---|---|---|---|---|---|---|---|
| (ccSTP/g) | (ka) | (ccSTP/cm/yr | (ka) | ||||
| 9 (Type 2a) | 2.30 | 6.65 | 135 | 3.35 | 9.85 | - | - |
| 16 (Type 1a) | 2.52 | 2.17 | 110 | 4.52 | 3.94 | - | - |
| Types 1a and 2a | 2.23 | 4.41 | 7.87 | 1.38 | 112 | 128 | |
| Types 1a and 2a | 1.67 | 2.17 | 84 | 63 | |||
| Types 1a and 2a | 2.52 | 6.65 | 126 | 193 | |||
| Type 1bmean | 6.70 | 5.76 | 335 | 1668 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heine, F.; Zosseder, K.; Einsiedl, F. Hydrochemical Zoning and Chemical Evolution of the Deep Upper Jurassic Thermal Groundwater Reservoir Using Water Chemical and Environmental Isotope Data. Water 2021, 13, 1162. https://doi.org/10.3390/w13091162
Heine F, Zosseder K, Einsiedl F. Hydrochemical Zoning and Chemical Evolution of the Deep Upper Jurassic Thermal Groundwater Reservoir Using Water Chemical and Environmental Isotope Data. Water. 2021; 13(9):1162. https://doi.org/10.3390/w13091162
Chicago/Turabian StyleHeine, Florian, Kai Zosseder, and Florian Einsiedl. 2021. "Hydrochemical Zoning and Chemical Evolution of the Deep Upper Jurassic Thermal Groundwater Reservoir Using Water Chemical and Environmental Isotope Data" Water 13, no. 9: 1162. https://doi.org/10.3390/w13091162