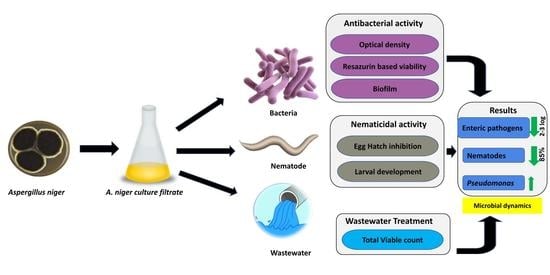
Aspergillus niger Culture Filtrate (ACF) Mediated Biocontrol of Enteric Pathogens in Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strain
2.2. Preparation of A. niger Culture Filtrate (ACF)
2.3. Bacterial Strains and Culture Conditions
2.4. Antibiotic Sensitivity Profiling of Bacterial Strains
2.5. Maintenance of C. elegans
2.6. Collection of Wastewater Sample
2.7. Determination of ACF Antimicrobial Activity
2.7.1. Spectrophotometric Assay
2.7.2. The Resazurin Based Fluorometric Assay
2.8. Effect of ACF on Biofilm Formation
2.9. Nematicidal Activity
2.9.1. Egg Hatch Assay
2.9.2. Larval Development Assay
2.10. Reduction of Enteric Pathogens in Wastewater
2.11. Statistical Analysis
3. Results
3.1. Antibiotic Sensitivity Profile (Antibiogram) of the Host Bacteria
3.2. Antimicrobial Activity of ACF on the Growth of Target Organisms
3.3. Effect of ACF on Biofilm Formation
3.4. Effect of A. niger Culture Filtrate on C. elegans Eggs and L1 Larvae
3.5. Effect of ACF against Potential and Presumptive Pathogens Present in Wastewater
3.6. Effect of Inactivated ACF against Potential and Presumptive Pathogens Present in Wastewater
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freeman, M.C.; Garn, J.V.; Sclar, G.D.; Boisson, S.; Medlicott, K.; Alexander, K.T.; Penakalapati, G.; Anderson, D.; Mahtani, A.G.; Grimes, J.E.T.; et al. The impact of sanitation on infectious disease and nutritional status: A systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2017, 220, 928–949. [Google Scholar] [CrossRef]
- Jahne, M.A.; Schoen, M.E.; Garland, J.L.; Ashbolt, N.J. Simulation of enteric pathogen concentrations in locally-collected greywater and wastewater for microbial risk assessments. Microb. Risk Anal. 2017, 5, 44–52. [Google Scholar] [CrossRef]
- Boopathy, R. Presence of Methicillin Resistant Staphylococcus aureus (MRSA) in sewage treatment plant. Bioresour. Technol. 2017, 240, 144–148. [Google Scholar] [CrossRef]
- Gerba, C.P. Transport of Pathogens in the Environment Questions and Problems References and Recommended Readings Environmentally Transmitted Pathogens. In Environmental Microbiology, 2nd ed.; Raina, M., Pepper, I.L., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 509–550. [Google Scholar]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Caccamo, F.M.; Torretta, V.; Rada, E.C.; Sorlini, S. Disinfection of wastewater by uv-based treatment for reuse in a circular economy perspective. Where are we at? Int. J. Environ. Res. Public Health 2021, 18, 77. [Google Scholar] [CrossRef]
- Owoseni, M.C.; Olaniran, A.O.; Okoh, A.I. Chlorine Tolerance and Inactivation of Escherichia coli recovered from wastewater treatment plants in the Eastern Cape, South Africa. Appl. Sci. 2017, 7, 810. [Google Scholar] [CrossRef]
- Chahal, C.; van den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and Particle Associations in Wastewater. Adv. Appl. Microbiol. 2016, 97, 63–119. [Google Scholar] [CrossRef] [PubMed]
- Al-Gheethi, A.A.; Efaq, A.N.; Bala, J.D.; Norli, I.; Abdel-Monem, M.O.; Ab Kadir, M.O. Removal of pathogenic bacteria from sewage-treated effluent and biosolids for agricultural purposes. Appl. Water Sci. 2018, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Salim, A.; Madhavan, A.; Babu, P.; Porayath, C.; Kesavan, M.; Hely, S.A.; Nair, B.G.; Pal, S. Bacteriophage-based control of biogenic hydrogen sulphide produced by multidrug resistant Salmonella enterica in synthetic sewage. J. Environ. Chem. Eng. 2021, 9, 105797. [Google Scholar] [CrossRef]
- Pini, A.K.; Geddes, P. Fungi Are Capable of Mycoremediation of River Water Contaminated by E. coli. Water. Air. Soil Pollut. 2020, 231, 83. [Google Scholar] [CrossRef]
- Periasamy, D.; Sundaram, A. A novel approach for pathogen reduction in wastewater treatment. J. Environ. Health Sci. Eng. 2013, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Sankaran, S.; Khanal, S.K.; Jasti, N.; Jin, B.; Pometto, A.L.; Van Leeuwen, J.H. Use of filamentous fungi for wastewater treatment and production of high value fungal byproducts: A review. Crit. Rev. Environ. Sci. Technol. 2010, 40, 400–449. [Google Scholar] [CrossRef]
- Upton, D.J.; McQueen-Mason, S.J.; Wood, A.J. An accurate description of Aspergillus niger organic acid batch fermentation through dynamic metabolic modelling. Biotechnol. Biofuels 2017, 10, 258. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, A.; Prasad, M.S.A.; Ramachandran, A.A.L.; Nair, B.; Madhavan, A.; Pal, S. Effect of Compost Derived Lytic Agents Against Enteric Pathogens in Wastewater. Pollut. Res. 2018, 37, 100–107. [Google Scholar]
- Vadlapudi, V.; Borah, N.; Yellusani, K.R.; Gade, S.; Reddy, P.; Rajamanikyam, M.; Vempati, L.N.S.; Gubbala, S.P.; Chopra, P.; Upadhyayula, S.M.; et al. Aspergillus Secondary Metabolite Database, a resource to understand the Secondary metabolome of Aspergillus genus. Sci. Rep. 2017, 7, 7325. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.F.; Mogensen, J.M.; Johansen, M.; Larsen, T.O.; Frisvad, J.C. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 2009, 395, 1225–1242. [Google Scholar] [CrossRef] [PubMed]
- Schuster, E.; Dunn-Coleman, N.; Frisvad, J.; Van Dijck, P. On the safety of Aspergillus niger—A review. Appl. Microbiol. Biotechnol. 2002, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Subhash, S.; Kuruvelil, A.B.; Aswathi, P.V.; Deepasree, K.; Navyamol, C.D.; Das Pv, N.; Prasad, P.; Nair, B.; Pal, S. Screening of nematicidal activities of biocontrol fungi aspergillus Niger and penicillium oxalicum using caenorhabditis elegans as model. Pollut. Res. 2018, 37, S121–S127. [Google Scholar]
- Ozdal, M.; Kurbanoglu, E.B. Citric Acid Production by Aspergillus niger from Agro-Industrial By-Products: Molasses and Chicken Feather Peptone. Waste Biomass Valorization 2019, 10, 631–640. [Google Scholar] [CrossRef]
- Iyyappan, J.; Bharathiraja, B.; Baskar, G.; Jayamuthunagai, J.; Barathkumar, S.; Anna Shiny, R. Malic acid production by chemically induced Aspergillus niger MTCC 281 mutant from crude glycerol. Bioresour. Technol. 2018, 251, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, X.; Liu, G.; Xu, Y.; Balan, V. Integrated production of gluconic acid and xylonic acid using dilute acid pretreated corn stover by two-stage fermentation. Biochem. Eng. J. 2018, 137, 18–22. [Google Scholar] [CrossRef]
- Yang, Y.; Song, W.; Ferrier, J.; Liu, F.; Csetenyi, L.; Gadd, G.M. Biorecovery of cobalt and nickel using biomass-free culture supernatants from Aspergillus niger. Appl. Microbiol. Biotechnol. 2020, 104, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Huang, J.; Mao, J.; Zhang, L.; He, C.; Chen, G.; Parkin, I.P.; Lai, Y. In vivo and in vitro efficient textile wastewater remediation by Aspergillus niger biosorbent. Nanoscale Adv. 2019, 1, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Feng, Y.Y. Feasibility of sewage sludge leached by Aspergillus Niger in land utilization. Polish J. Environ. Stud. 2016, 25, 405–412. [Google Scholar] [CrossRef]
- Ahmad, Z.; Butt, M.S.; Ahmed, A.; Riaz, M.; Sabir, S.M.; Farooq, U.; Rehman, F.U. Effect of Aspergillus niger xylanase on dough characteristics and bread quality attributes. J. Food Sci. Technol. 2014, 51, 2445–2453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Mao, Y.; Zhao, F.; Zhang, X.-X.; Ju, F.; Ye, L.; Wang, Y.; Li, B.; Ren, H.; Zhang, T. Free-living bacteria and potential bacterial pathogens in sewage treatment plants. Appl. Microbiol. Biotechnol. 2018, 102, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Porayath, C.; Salim, A.; Palillam Veedu, A.; Babu, P.; Nair, B.; Madhavan, A.; Pal, S. Characterization of the bacteriophages binding to human matrix molecules. Int. J. Biol. Macromol. 2018, 110, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Azim, M.K. Characterization of the nematicidal activity of natural honey. J. Agric. Food Chem. 2012, 60, 7428–7434. [Google Scholar] [CrossRef]
- Man, Y.B.; Chow, K.L.; Cheng, Z.; Kang, Y.; Wong, M.H. Profiles and removal efficiency of organochlorine pesticides with emphasis on DDTs and HCHs by two different sewage treatment works. Environ. Technol. Innov. 2018, 9, 220–231. [Google Scholar] [CrossRef]
- Microbiology, A. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory. Susceptibility testing: Accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration. Microbiol. Appl. 2016, 88, 784–790. [Google Scholar] [CrossRef]
- Campbell, J. High-Throughput Assessment of Bacterial Growth Inhibition by Optical Density Measurements. Curr. Protoc. Chem. Biol. 2010, 2, 195–208. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, K.; McVey, A.F.; Clark, I.B.N.; Swain, P.S.; Pilizota, T. General calibration of microbial growth in microplate readers. Sci. Rep. 2016, 6, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Haase, H.; Jordan, L.; Keitel, L.; Keil, C.; Mahltig, B. Comparison of methods for determining the effectiveness of antibacterial functionalized textiles. PLoS ONE 2017, 12, e0188304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haripriyan, J.; Omanakuttan, A.; Menon, N.D.; Vanuopadath, M.; Nair, S.S.; Corriden, R.; Nair, B.G.; Nizet, V.; Kumar, G.B. Clove Bud Oil Modulates Pathogenicity Phenotypes of the Opportunistic Human Pathogen Pseudomonas aeruginosa. Sci. Rep. 2018, 8, 3437. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.; Poornendhu, S.J.; Salim, A.; Madhavan, A.; Nair, B.; Pal, S. Resazurin based redox dye as an indicator for monitoring wastewater biological activity. Pollut. Res. 2018, 37, S115–S120. [Google Scholar]
- Prakash, V.; Sreetha, H.; Poornima, K.H.; Lakshmimol, K.N.; Regma, R.; Fathima, H.; Vishnu, T.V.; Venu, S.; Nair, B.G.; Pal, S. Antagonistic effects of bacteriocins from Lactobacillus Spp. against enteric pathogens. Pollut. Res. 2018, 37, 128–134. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef]
- Bogdanova, K.; Röderova, M.; Kolar, M.; Langova, K.; Dusek, M.; Jost, P.; Kubelkova, K.; Bostik, P.; Olsovska, J. Antibiofilm activity of bioactive hop compounds humulone, lupulone and xanthohumol toward susceptible and resistant staphylococci. Res. Microbiol. 2018, 169, 127–134. [Google Scholar] [CrossRef]
- Günther, F.; Scherrer, M.; Kaiser, S.J.; DeRosa, A.; Mutters, N.T. Comparative testing of disinfectant efficacy on planktonic bacteria and bacterial biofilms using a new assay based on kinetic analysis of metabolic activity. J. Appl. Microbiol. 2017, 122, 625–633. [Google Scholar] [CrossRef]
- Jang, J.Y.; Choi, Y.H.; Shin, T.S.; Kim, T.H.; Shin, K.S.; Park, H.W.; Kim, Y.H.; Kim, H.; Choi, G.J.; Jang, K.S.; et al. Biological control of Meloidogyne incognita by Aspergillus niger F22 producing oxalic acid. PLoS ONE 2016, 11, e0156230. [Google Scholar] [CrossRef] [Green Version]
- Sant’anna, V.; Vommaro, R.C.; de Souza, W. Caenorhabditis elegans as a model for the screening of anthelminthic compounds: Ultrastructural study of the effects of albendazole. Exp. Parasitol. 2013, 135, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chaweeborisuit, P.; Suriyonplengsaeng, C.; Suphamungmee, W.; Sobhon, P.; Meemon, K. Nematicidal effect of plumbagin on Caenorhabditis elegans: A model for testing a nematicidal drug. Z. Naturforsch.-Sect. C J. Biosci. 2016, 71, 121–131. [Google Scholar] [CrossRef]
- Moges, F.; Endris, M.; Belyhun, Y.; Worku, W. Isolation and characterization of multiple drug resistance bacterial pathogens from waste water in hospital and non-hospital environments, Northwest Ethiopia. BMC Res. Notes 2014, 7, 2–7. [Google Scholar] [CrossRef]
- Kabiru, L.M.; Bello, M.; Kabir, J.; Grande, L.; Morabito, S. Detection of pathogenic Escherichia coli in samples collected at an abattoir in Zaria, Nigeria and at different points in the surrounding environment. Int. J. Environ. Res. Public Health 2015, 12, 679–691. [Google Scholar] [CrossRef] [Green Version]
- Ng, C.; Goh, S.G.; Saeidi, N.; Gerhard, W.A.; Gunsch, C.K.; Gin, K.Y.H. Occurrence of Vibrio species, beta-lactam resistant Vibrio species, and indicator bacteria in ballast and port waters of a tropical harbor. Sci. Total Environ. 2018, 610–611, 651–656. [Google Scholar] [CrossRef]
- Ben Said, M.; Abbassi, M.S.; Gómez, P.; Ruiz-Ripa, L.; Sghaier, S.; Ibrahim, C.; Torres, C.; Hassen, A. Staphylococcus aureus isolated from wastewater treatment plants in Tunisia: Occurrence of human and animal associated lineages. J. Water Health 2017, 15, 638–643. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, L.G.; Ferreira, L.G.R.; Nascimento, A.M.A.; Reis, M.D.P.; Dias, M.F.; Lima, W.G.; Paiva, M.C. Antibiotic resistance profile and occurrence of AmpC between Pseudomonas aeruginosa isolated from a domestic full-scale WWTP in southeast Brazil. Water Sci. Technol. 2017, 2017, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirimura, K.; Yoda, M.; Usami, S. Cloning and expression of the cDNA encoding an alternative oxidase gene from Aspergillus niger WU-2223L. Curr. Genet. 1999, 34, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Hattori, T.; Honda, Y.; Kirimura, K. Oxalic acid production by citric acid-producing Aspergillus niger overexpressing the oxaloacetate hydrolase gene oahA. J. Ind. Microbiol. Biotechnol. 2014, 41, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Zaher, E.H.F.; Abou-Zeid, A.M.; Mostafa, A.A.; Arif, D.M. Industrial oil wastewater treatment by free and immobilized Aspergillus niger KX759617 and the possibility of using it in crop irrigation. Rend. Lincei 2017, 28, 93–103. [Google Scholar] [CrossRef]
- Assadia, M.M.; Jahangirib, M.R. Textile wastewater treatment by Aspergillus niger. Desalination 2001, 141, 1–6. [Google Scholar] [CrossRef]
- Pu, S.; Ma, H.; Deng, D.; Xue, S.; Zhu, R.; Zhou, Y. Isolation, identification, and characterization of an Aspergillus niger bioflocculant-producing strain using potato starch wastewater as nutrilite and its application. PLoS ONE 2018, 13, e0190236. [Google Scholar]
- Svahn, K.S.; Göransson, U.; El-Seedi, H.; Bohlin, L.; Larsson, D.G.J.; Olsen, B.; Chryssanthou, E. Antimicrobial activity of filamentous fungi isolated from highly antibiotic-contaminated river sediment. Infect. Ecol. Epidemiol. 2012, 2, 11591. [Google Scholar] [CrossRef]
- Jayalekshmi, H.; Omanakuttan, A.; Pandurangan, N.; Vargis, V.S.; Maneesh, M.; Nair, B.G.; Kumar, G.B. Clove bud oil reduces kynurenine and inhibits pqs A gene expression in P. aeruginosa. Appl. Microbiol. Biotechnol. 2016, 100, 3681–3692. [Google Scholar] [CrossRef]
- Lopes, B.C.; Machado, E.C.; Rodrigues, H.F.; Leal, C.D.; de Araújo, J.C.; Teixeira de Matos, A. Effect of alkaline treatment on pathogens, bacterial community and antibiotic resistance genes in different sewage sludges for potential agriculture use. Environ. Technol. 2020, 41, 529–538. [Google Scholar] [CrossRef]
- Bindiya, E.S.; Tina, K.J.; Sasidharan, R.S.; Bhat, S.G. BaCf3: Highly thermostable bacteriocin from Bacillus amyloliquefaciens BTSS3 antagonistic on food-borne pathogens. 3 Biotech 2019, 9, 136. [Google Scholar] [CrossRef]
- Dabrowska, J.; Zdybel, J.; Karamon, J.; Kochanowski, M.; Stojecki, K.; Cencek, T.; Kłapeć, T. Assessment of viability of the nematode eggs (Ascaris, Toxocara, Trichuris) in sewage sludge with the use of LIVE/DEAD bacterial viability kit. Ann. Agric. Environ. Med. 2014, 21, 35–41. [Google Scholar] [PubMed]
- Ensink, J.H.J.; Blumenthal, U.J.; Brooker, S. Wastewater quality and the risk of intestinal nematode infection in sewage farming families in Hyderabad, India. Am. J. Trop. Med. Hyg. 2008, 79, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.R.; Luciani, G.M.; Musso, G.; Bagg, R.; Yeo, M.; Zhang, Y.; Rajendran, L.; Glavin, J.; Hunter, R.; Redman, E.; et al. Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat. Commun. 2015, 6, 7485. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaibani, A.B.A.; Al-Shakarchi, F.I.; Ameen, R.S. Extraction and Characterization of Antibacterial Compound from Aspergillus niger. J. Al-Nahrain Univ. Sci. 2013, 16, 167–174. [Google Scholar] [CrossRef]
- Nwakanma, C.; Njoku, E.N.; Pharamat, T. Antimicrobial Activity of Secondary Metabolites of Fungi Isolated from Leaves of Bush Mango. J. Next Gener. Seq. Appl. 2016, 3, 2. [Google Scholar] [CrossRef]
- Kalyani, P.; Hemalatha, K.P.J. In Vitro Antimicrobial Potential of Aspergillus niger. Int. J. ChemTech Res. 2017, 10, 430–435. [Google Scholar]
- Li, N.; Xia, Y.; He, X.; Li, W.; Yuan, L.; Wu, X.; Qin, Y.; Yuan, R.; Gong, X. Glucose addition enhanced the advanced treatment of coking wastewater. Water 2021, 13, 3365. [Google Scholar] [CrossRef]
- Tharuvana, D.; Sundaresh, A.; Sreelakshmi, V.J.; Das, A.; Nair, B.; Madhavan, A.; Pal, S. Sand and charcoal as immobilization matrices of phages and probiotics for wastewater treatment. Pollut. Res. 2018, 37, S108–S114. [Google Scholar]
- Salim, A.; Sindhu Shetty, K.; Febin, H.; Sameed, N.; Pal, S.; Nair, B.G.; Madhavan, A. Lytics broadcasting system: A novel approach to disseminate bacteriophages for disinfection and biogenic hydrogen sulphide removal tested in synthetic sewage. Results Eng. 2022, 13, 100314. [Google Scholar] [CrossRef]
- Cray, J.A.; Bell, A.N.W.; Bhaganna, P.; Mswaka, A.Y.; Timson, D.J.; Hallsworth, J.E. The biology of habitat dominance; can microbes behave as weeds? Microb. Biotechnol. 2013, 6, 453–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Bacterial Strains | Antibiotics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| P | C | MET | TE | CAZ | AMP | CIP | AMX | CX | |
| E. coli MDR | − | − | − | − | − | − | − | − | − |
| K. pneumoniae | − | + | − | − | + | − | + | − | − |
| K. variicola | − | + | − | + | + | − | + | − | + |
| P. aeruginosa | − | + | − | − | − | − | + | − | + |
| V. cholerae | − | + | − | + | − | − | + | − | + |
| S. enterica | − | + | − | − | + | + | + | − | + |
| S. dysenteriae | − | − | − | − | + | − | + | − | + |
| S. aureus | + | + | + | + | + | − | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subhash, S.; Babu, P.; Vijayakumar, A.; Suresh, R.A.; Madhavan, A.; Nair, B.G.; Pal, S. Aspergillus niger Culture Filtrate (ACF) Mediated Biocontrol of Enteric Pathogens in Wastewater. Water 2022, 14, 119. https://doi.org/10.3390/w14010119
Subhash S, Babu P, Vijayakumar A, Suresh RA, Madhavan A, Nair BG, Pal S. Aspergillus niger Culture Filtrate (ACF) Mediated Biocontrol of Enteric Pathogens in Wastewater. Water. 2022; 14(1):119. https://doi.org/10.3390/w14010119
Chicago/Turabian StyleSubhash, Suja, Pradeesh Babu, Amrutha Vijayakumar, Reshma Alookaran Suresh, Ajith Madhavan, Bipin Gopalakrishnan Nair, and Sanjay Pal. 2022. "Aspergillus niger Culture Filtrate (ACF) Mediated Biocontrol of Enteric Pathogens in Wastewater" Water 14, no. 1: 119. https://doi.org/10.3390/w14010119