Enhanced Ozone Oxidation by a Novel Fe/Mn@γ−Al2O3 Nanocatalyst: The Role of Hydroxyl Radical and Singlet Oxygen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Ozonation Procedure
2.4. Analytic Methods
3. Results
3.1. Characterization
3.2. Degradation of DMP and 1−NP
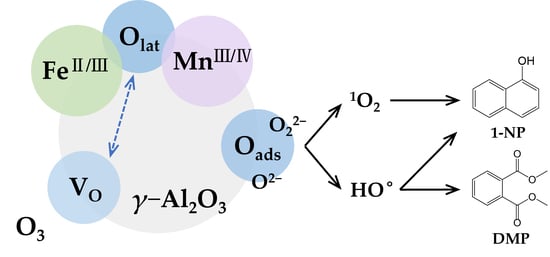
3.3. Catalytic Ozonation Mechanism
3.4. Proposed Degradation Pathways of DMP and 1−NP
3.5. Effects of Water Matrix
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Pearce, C.I.; Lloyd, J.R.; Guthrie, J.T. The removal of colour from textile wastewater using whole bacterial cells: A review. Dye. Pigment. 2003, 58, 179–196. [Google Scholar] [CrossRef]
- McMullan, G.; Meehan, C.; Conneely, A.; Kirby, N.; Robinson, T.; Nigam, P.; Banat, I.; Marchant, R.; Smyth, W.F. Microbial decolourisation and degradation of textile dyes. Appl. Microbiol. Biotechnol. 2001, 56, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Lara-Ramos, J.A.; Figueroa Angulo, M.A.; Machuca-Martínez, F.; Mueses, M.A. Sensitivity Analysis of the Catalytic Ozonation under Different Kinetic Modeling Approaches in the Diclofenac Degradation. Water 2021, 13, 3003. [Google Scholar] [CrossRef]
- Beltrán, F.J.; Rivas, J.; Montero−De−Espinosa, R. Iron type catalysts for the ozonation of oxalic acid in water. Water Res. 2005, 39, 3553–3564. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yang, L. The adsorption of basic dyes from aqueous solution on modified peat–resin particle. Water Res. 2003, 37, 1535–1544. [Google Scholar] [CrossRef]
- Kumar, M.; Sridhari, T.; Bhavani, K.; Dutta, P. Trends in color removal from textile mill effluents. Colourage 1998, 45, 25–34. [Google Scholar]
- Zugic, B.; Wang, L.C.; Heine, C.; Zakharov, D.N.; Lechner, B.A.J.; Stach, E.A.; Biener, J.; Salmeron, M.; Madix, R.J.; Friend, C.M. Dynamic restructuring drives catalytic activity on nanoporous gold−silver alloy catalysts. Nat. Mater. 2017, 16, 558–564. [Google Scholar] [CrossRef]
- Ghuge, S.P.; Saroha, A.K. Catalytic ozonation of dye industry effluent using mesoporous bimetallic Ru−Cu/SBA−15 catalyst. Process. Saf. Environ. Prot. 2018, 118, 125–132. [Google Scholar] [CrossRef]
- Tang, Y.M.; Pan, Z.Q.; Li, L.S. pH−insusceptible cobalt−manganese immobilizing mesoporous siliceous MCM−41 catalyst for ozonation of dimethyl phthalate. J. Colloid Interf. Sci. 2017, 508, 196–202. [Google Scholar] [CrossRef]
- Lu, X.H.; Zhang, Q.Y.; Yang, W.Q.; Li, X.K.; Zeng, L.X.; Li, L.S. Catalytic ozonation of 2,4−dichlorophenoxyacetic acid over novel Fe−Ni/AC. Rsc. Adv. 2015, 5, 10537–10545. [Google Scholar] [CrossRef]
- Xiong, Z.K.; Cao, J.Y.; Lai, B.; Yang, P. Comparative study on degradation of p−nitrophenol in aqueous solution by mFe/Cu/O3 and mFe0/O3 processes. J. Ind. Eng. Chem. 2018, 59, 196–207. [Google Scholar] [CrossRef]
- Ma, J.; Sui, M.; Zhang, T.; Guan, C. Effect of pH on MnOx/GAC catalyzed ozonation for degradation of nitrobenzene. Water Res. 2005, 39, 779–786. [Google Scholar] [CrossRef]
- Einaga, H.; Futamura, S. Catalytic oxidation of benzene with ozone over alumina−supported manganese oxides. J. Catal. 2004, 227, 304–312. [Google Scholar] [CrossRef]
- Beltran, F.J.; Rivas, F.J.; Montero−De−Espinosa, R. Ozone−Enhanced Oxidation of Oxalic Acid in Water with Cobalt Catalysts. 1. Homogeneous Catalytic Ozonation. Ind. Eng. Chem. Res. 2003, 42, 3210–3217. [Google Scholar] [CrossRef]
- Pines, D.S.; Reckhow, D.A. Effect of Dissolved Cobalt(II) on the Ozonation of Oxalic Acid. Environ. Sci. Technol. 2002, 36, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Graham, N.J.D. Degradation of atrazine by manganese−catalysed ozonation—Influence of radical scavengers. Water Res. 2000, 34, 3822–3828. [Google Scholar] [CrossRef]
- Cao, H.; Xing, L.; Wu, G.; Xie, Y.; Shi, S.; Zhang, Y.; Minakata, D.; Crittenden, J.C. Promoting effect of nitration modification on activated carbon in the catalytic ozonation of oxalic acid. Appl. Catal. B Environ. 2014, 146, 169–176. [Google Scholar] [CrossRef]
- Wang, Y.X.; Chen, L.L.; Cao, H.B.; Chi, Z.X.; Chen, C.M.; Duan, X.G.; Xie, Y.B.; Qi, F.; Song, W.Y.; Liu, J.; et al. Role of oxygen vacancies and Mn sites in hierarchical Mn2O3/LaMnO3−delta perovskite composites for aqueous organic pollutants decon−tamination. Appl. Catal. B−Environ. 2019, 245, 546–554. [Google Scholar] [CrossRef]
- Xu, B.J.; Xiao, T.C.; Yan, Z.F.; Sun, X.; Sloan, J.; Gonzalez−Cortes, S.L.; Alshahrani, F.; Green, M.L.H. Synthesis of mesoporous alumina with highly thermal stability using glucose template in aqueous system. Micropor. Mesopor. Mat. 2006, 91, 293–295. [Google Scholar] [CrossRef]
- Cho, K.; Rana, B.S.; Cho, D.W.; Beum, H.T.; Kim, C.H.; Kim, J.N. Catalytic removal of naphthenic acids over Co−Mo/gamma−Al2O3 catalyst to reduce total acid number (TAN) of highly acidic crude oil. Appl. Catal. A−Gen. 2020, 606, 117835. [Google Scholar] [CrossRef]
- Teng, Y.; Wang, X.-D.; Liao, J.-F.; Li, W.-G.; Chen, H.-Y.; Dong, Y.-J.; Kuang, D.-B. Atomically Thin Defect−Rich Fe−Mn−O Hybrid Nanosheets as High Efficient Electrocatalyst for Water Oxidation. Adv. Funct. Mater. 2018, 28, 28. [Google Scholar] [CrossRef]
- Naskar, M.K. Hydrothermal Synthesis of Petal−Like Alumina Flakes. J. Am. Ceram. Soc. 2009, 92, 2392–2395. [Google Scholar] [CrossRef]
- Zhu, L.; Pu, S.; Liu, K.; Zhu, T.; Lu, F.; Li, J. Preparation and characterizations of porous γ−Al2O3 nanoparticles. Mater. Lett. 2012, 83, 73–75. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Zheng, X.S.; Li, D.T. Ozonation of naphthalene sulfonic acids in aqueous solutions. Part I: Elimination of COD, TOC and increase of their biodegradability. Water Res. 2002, 36, 1237–1243. [Google Scholar]
- Wang, P.; Matta, H.; Kuo, C.-H. Kinetics of ozonation of naphthalene and anthracene. J. Chin. I. Ch. E 1991, 22, 365–371. [Google Scholar]
- Hoigné, J.; Bader, H.; Haag, W.; Staehelin, J. Rate constants of reactions of ozone with organic and inorganic compounds in water—III. Inorganic compounds and radicals. Water Res. 1985, 19, 993–1004. [Google Scholar] [CrossRef]
- Huber, M.M.; Canonica, S.; Park, G.-Y.; von Gunten, U. Oxidation of Pharmaceuticals during Ozonation and Advanced Oxidation Processes. Environ. Sci. Technol. 2003, 37, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, C.; Ma, J.; Tian, H.; Qiang, Z. Surface hydroxyl groups of synthetic α−FeOOH in promoting OH generation from aqueous ozone: Property and activity relationship. Appl. Catal. B Environ. 2008, 82, 131–137. [Google Scholar] [CrossRef]
- Ma, J.; Graham, N.J. Degradation of atrazine by manganese−catalysed ozonation: Influence of humic substances. Water Res. 1999, 33, 785–793. [Google Scholar] [CrossRef]
- Sui, M.; Liu, J.; Sheng, L. Mesoporous material supported manganese oxides (MnOx/MCM−41) catalytic ozonation of nitrobenzene in water. Appl. Catal. B Environ. 2011, 106, 195–203. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Cao, H.; Zhao, H.; Xie, Y. Reactive Oxygen Species and Catalytic Active Sites in Heterogeneous Catalytic Ozonation for Water Purification. Environ. Sci. Technol. 2020, 54, 5931–5946. [Google Scholar] [CrossRef]
- Yao, C.C.D.; Haag, W.R. Rate constants for direct reactions of ozone with several drinking water contaminants. Water Res. 1991, 25, 761–773. [Google Scholar]
- Lei, Y.; Lu, J.; Zhu, M.; Xie, J.; Peng, S.; Zhu, C. Radical chemistry of diethyl phthalate oxidation via UV/peroxymonosulfate process: Roles of primary and secondary radicals. Chem. Eng. J. 2020, 379, 122339. [Google Scholar] [CrossRef]
- Kanodia, S.; Madhaven, V.; Schuler, R. Oxidation of naphthalene by radiolytically produced OH radicals. Int. J. Radiat. Appl. Instrumentation. Part. C. Radiat. Phys. Chem. 1988, 32, 661–664. [Google Scholar] [CrossRef]
- Darmanyan, A.P.; Moger, G. Interaction of singlet oxygen with nitroso− and phenol−type inhibitors. Magy. Kem. F. 1988, 94, 282–284. [Google Scholar]
- Zuo, Z.; Cai, Z.; Katsumura, Y.; Chitose, N.; Muroya, Y. Reinvestigation of the acid–base equilibrium of the (bi)carbonate radical and pH dependence of its reactivity with inorganic reactants. Radiat. Phys. Chem. 1999, 55, 15–23. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Sun, Z.; Ma, J. Novel Relationship between Hydroxyl Radical Initiation and Surface Group of Ceramic Honeycomb Supported Metals for the Catalytic Ozonation of Nitrobenzene in Aqueous Solution. Environ. Sci. Technol. 2009, 43, 4157–4163. [Google Scholar] [CrossRef]
- Ervens, B.; Gligorovski, S.; Herrmann, H. Temperature−dependent rate constants for hydroxyl radical reactions with organic compounds in aqueous solutions. Phys. Chem. Chem. Phys. 2003, 5, 1811–1824. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, G.; Li, X.; Lu, X. Removal of rare earth elements by MnFe2O4 based mesoporous adsorbents: Synthesis, isotherms, kinetics, thermodynamics. J. Alloys Compd. 2021, 856, 158185. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, G.; Hu, F.; Li, X. Synthesis of mesoporous magnetic MnFe2O4@CS−SiO2 microsphere and its adsorption performance of Zn2+ and MB studies. J. Environ. Manag. 2020, 263, 110377. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, Z.; Liu, J.; Deng, Y.; Zhang, D.; Du, P.; Zhang, S.; Lu, X. Novel Fe−Mn−O nanosheets/wood carbon hybrid with tunable surface properties as a superior catalyst for Fenton−like oxidation. Appl. Catal. B Environ. 2019, 259, 118058. [Google Scholar] [CrossRef]
- Shen, T.; Su, W.; Yang, Q.; Ni, J.; Tong, S. Synergetic mechanism for basic and acid sites of MgMxOy (M = Fe, Mn) double oxides in catalytic ozonation of p−hydroxybenzoic acid and acetic acid. Appl. Catal. B Environ. 2020, 279, 119346. [Google Scholar] [CrossRef]
- Guan, S.; An, L.; Ashraf, S.; Zhang, L.; Liu, B.; Fan, Y.; Li, B. Oxygen vacancy excites Co3O4 nanocrystals embedded into carbon nitride for accelerated hydrogen generation. Appl. Catal. B Environ. 2020, 269, 118775. [Google Scholar] [CrossRef]
- Yan, H.; Lu, P.; Pan, Z.; Wang, X.; Zhang, Q.; Li, L. Ce/SBA−15 as a heterogeneous ozonation catalyst for efficient minerali−zation of dimethyl phthalate. J. Mol. Catal. A Chem. 2013, 377, 57–64. [Google Scholar] [CrossRef]
- Orge, C.A.; Órfão, J.J.; Pereira, M.F.; de Farias, A.M.D.; Fraga, M.A. Ceria and cerium-based mixed oxides as ozonation catalysts. Chem. Eng. J. 2012, 200-202, 499–505. [Google Scholar] [CrossRef]
- 4Rui, C.M.; Quinta−Ferreira, R.M. Catalytic ozonation of phenolic acids over a Mn–Ce–O catalyst. Appl. Catal. B Environ. Ment. 2009, 90, 268–277. [Google Scholar]
- Li, L.; Ye, W.; Zhang, Q.; Sun, F.; Li, X. Catalytic ozonation of dimethyl phthalate over cerium supported on activated car−bon. J. Hazard. Mater. 2009, 170, 411–416. [Google Scholar] [CrossRef]
- He, C.; Wang, Y.; Li, Z.; Huang, Y.; Liao, Y.; Xia, D.; Lee, S.-C. Facet Engineered α−MnO2 for Efficient Catalytic Ozonation of Odor CH3SH: Oxygen Vacancy−Induced Active Centers and Catalytic Mechanism. Environ. Sci. Technol. 2020, 54, 12771–12783. [Google Scholar] [CrossRef]
- He, D.; Wan, G.; Hao, H.; Chen, D.; Lu, J.; Zhang, L.; Liu, F.; Zhong, L.; He, S.; Luo, Y. Microwave−assisted rapid synthesis of CeO2 nanoparticles and its desulfuri−zation processes for CH3SH catalytic decomposition. Chem. Eng. J. Lausanne 2016, 289, 161–169. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Zhang, Y.; Ding, Y.; Bi, Y. Ultrathin FeOOH Nanolayers with Abundant Oxygen Vacancies on BiVO4 Photoanodes for Efficient Water Oxidation. Angew. Chem. Int. Ed. 2018, 57, 2248–2252. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Wang, Z.; Zhang, K.; He, B.; Liu, Y.; Zhou, W.; Xu, J.; Wang, Q.; Zhao, L. Oxygen vacancies−rich Ce0.9Gd0.1O2−δ decorated Pr0.5Ba0.5CoO3−δ bifunctional catalyst for efficient and long−lasting rechargeable Zn−air batteries. Appl. Catal. B Environ. 2020, 266, 118656. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, X.; Shi, Y.; Zhang, H. Natural Fe−bearing manganese ore facilitating bioelectro−activation of peroxymonosulfate for bisphenol A oxidation. Chem. Eng. J. 2018, 354, 1120–1131. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Kasprzyk−Hordern, B. Catalytic ozonation of chlorinated VOCs on ZSM−5 zeolites and alumina: Formation of chlorides. Appl. Catal. B Environ. 2017, 200, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Ikhlaq, A.; Brown, D.; Kasprzyk−Hordern, B. Catalytic ozonation for the removal of organic contaminants in water on ZSM−5 zeolites. Appl. Catal. B Environ. 2014, 154–155, 110–122. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.; Kasprzyk−Hordern, B. Mechanisms of catalytic ozonation: An investigation into superoxide ion radical and hydrogen peroxide formation during catalytic ozonation on alumina and zeolites in water. Appl. Catal. B Environ. 2013, 129, 437–449. [Google Scholar] [CrossRef]
- Bing, J.; Hu, C.; Nie, Y.; Yang, M.; Qu, J. Mechanism of Catalytic Ozonation in Fe2O3/Al2O3@SBA−15 Aqueous Suspension for Destruction of Ibuprofen. Environ. Sci. Technol. 2015, 49, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hu, C.; Nie, Y.; Qu, J. Catalytic Ozonation of Selected Pharmaceuticals over Mesoporous Alumina—Supported Manganese Oxide. Environ. Sci. Technol. 2009, 43, 2525–2529. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhong, S.; Dai, Y.; Liu, C.-C.; Zhang, H.J. Effect of MnO2 Phase Structure on the Oxidative Reactivity toward Bisphenol A Degradation. Environ. Sci. Technol. 2018, 52, 11309–11318. [Google Scholar] [CrossRef] [PubMed]
- Roman, P.; Veltman, R.; Bijmans, M.F.M.; Keesman, K.J.; Janssen, A.J.H. Effect of Methanethiol Concentration on Sulfur Production in Biological Desulfurization Systems under Haloalkaline Conditions. Environ. Sci. Technol. 2015, 49, 9212–9221. [Google Scholar] [CrossRef]
- Buehler, R.E.; Staehelin, J.; Hoigne, J. Ozone decomposition in water studied by pulse radiolysis. 1. Perhydroxyl (HO2)/hyperoxide (O2−) and HO3/O3− as intermediates. J. Phys. Chem. 1984, 88, 2560–2564. [Google Scholar] [CrossRef]
- Long, L.; Zhao, J.; Yang, L.; Fu, M.; Wu, J.; Huang, B.; Ye, D. Room Temperature Catalytic Ozonation of Toluene over MnO2/Al2O3. Chin. J. Catal. 2011, 32, 904–916. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Zhang, Y.; Mao, S.; Wu, D.; Chu, H. Highly efficient degradation of dimethyl phthalate from Cu(II) and dimethyl phthalate wastewater by EDTA enhanced ozonation: Performance, intermediates and mechanism. J. Hazard. Mater. 2019, 366, 378–385. [Google Scholar] [CrossRef]
- An, T.; Gao, Y.; Li, G.; Kamat, P.V.; Peller, J.; Joyce, M.V. Kinetics and Mechanism of •OH Mediated Degradation of Dimethyl Phthalate in Aqueous Solution: Experimental and Theoretical Studies. Environ. Sci. Technol. 2014, 48, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, D.; Liu, Y.; Wang, H.; Zhang, C.; Huang, H.; He, Y.; Chen, X.; Du, Z.; Zheng, X. Potential anticancer activity of curcumin analogs containing sulfone on human cancer cells. Arch. Biol. Sci. 2016, 68, 125–133. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Li, J.; Wang, X. Ozone degradation of 1−naphthol on multiwalled carbon nanotubes/iron oxides and recycling of the adsorbent. Chem. Eng. J. 2015, 262, 1303–1310. [Google Scholar] [CrossRef]
- Acero, J.L.; Haderlein, S.B.; Schmidt, T.C.; Suter, M.J.-F.; von Gunten, U. MTBE Oxidation by Conventional Ozonation and the Combination Ozone/Hydrogen Peroxide: Efficiency of the Processes and Bromate Formation. Environ. Sci. Technol. 2001, 35, 4252–4259. [Google Scholar] [CrossRef]
- Madhavan, D.; Pitchumani, K. Photoreactions in clay media: Singlet oxygen oxidation of electron−rich substrates mediated by clay−bound dyes. J. Photochem. Photobiol. A Chem. 2002, 153, 205–210. [Google Scholar] [CrossRef]
- Kim, K.-J.; Hamill, W.H. Pulse radiolysis of concentrated aqueous solutions of chloride, iodide, and persulfate ions. J. Phys. Chem. 1976, 80, 2325–2330. [Google Scholar] [CrossRef]
- Jayson, G.G.; Parsons, B.J.; Swallow, A.J. Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1973, 69, 1597–1607. [Google Scholar] [CrossRef]
- Buxton, G.V.; Wood, N.D.; Dyster, S. Ionisation constants of °OH and HO°2 in aqueous solution up to 200 °C. A pulse radiolysis study. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 1113–1121. [Google Scholar] [CrossRef]
- Kasprzyk−Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, M.; Li, S.; Xia, D.; Tang, Z.; Hu, S.; Ye, S.; Sun, M.; He, C.; Shu, D. Efficient catalytic activity and bromate minimization over lattice oxygen−rich MnOOH nanorods in catalytic ozonation of bromide−containing organic pollutants: Lattice oxygen−directed redox cycle and bromate reduction. J. Hazard. Mater. 2021, 410, 124545. [Google Scholar] [CrossRef]
- Shahmahdi, N.; Dehghanzadeh, R.; Aslani, H.; Shokouhi, S.B. Performance evaluation of waste iron shavings (Fe0) for catalytic ozonation in removal of sulfamethoxazole from municipal wastewater treatment plant effluent in a batch mode pilot plant. Chem. Eng. J. 2020, 383, 123093. [Google Scholar] [CrossRef]





| Compound | Rate Constant (M−1 s−1) | Exp. k (s−1) | Est. k * (s−1) | ||
|---|---|---|---|---|---|
| O3 | HO° | 1O2 | |||
| DMP | 0.2 [33] | 3.7 × 109 # | <103 [34] | 3.0 × 10−3 | 2.1 × 10−3 |
| 1−NP | ~102 $ | 1.3 × 1010 [35] | 7.6 × 106 [36] | 3.3 × 10−2 | 2.2 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Luo, X.; Hu, Y. Enhanced Ozone Oxidation by a Novel Fe/Mn@γ−Al2O3 Nanocatalyst: The Role of Hydroxyl Radical and Singlet Oxygen. Water 2022, 14, 19. https://doi.org/10.3390/w14010019
Liang C, Luo X, Hu Y. Enhanced Ozone Oxidation by a Novel Fe/Mn@γ−Al2O3 Nanocatalyst: The Role of Hydroxyl Radical and Singlet Oxygen. Water. 2022; 14(1):19. https://doi.org/10.3390/w14010019
Chicago/Turabian StyleLiang, Chen, Xinhao Luo, and Yongyou Hu. 2022. "Enhanced Ozone Oxidation by a Novel Fe/Mn@γ−Al2O3 Nanocatalyst: The Role of Hydroxyl Radical and Singlet Oxygen" Water 14, no. 1: 19. https://doi.org/10.3390/w14010019