Metacommunity Concepts Provide New Insights in Explaining Zooplankton Spatial Patterns within Large Floodplain Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Statistical Analysis
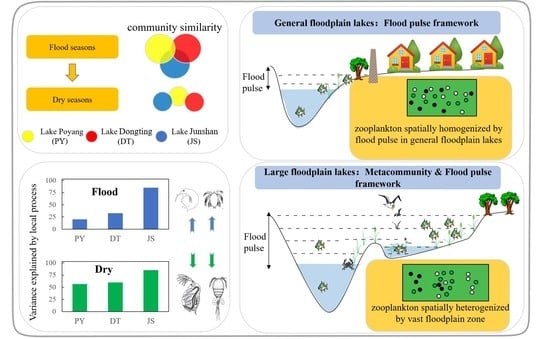
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Simoes, N.R.; Dias, J.D.; Leal, C.M.; Magalhaes Braghin, L.d.S.; Lansac-Toha, F.A.; Bonecker, C.C. Floods control the influence of environmental gradients on the diversity of zooplankton communities in a neotropical floodplain. Aquat. Sci. 2013, 75, 607–617. [Google Scholar] [CrossRef]
- Junk, W.J.; Bayley, P.B.; Sparks, R.E. The flood pulse concept in river-floodplain systems. Can. Spec. Publ. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Cardinale, B.J. Biodiversity improves water quality through niche partitioning. Nature 2011, 472, 86-U113. [Google Scholar] [CrossRef] [PubMed]
- Bini, L.M.; Tundisi, J.G.; Matsumura-Tundisi, T.; Matheus, C.E. Spatial variation of zooplankton groups in a tropical reservoir (Broa Reservoir, São Paulo State-Brazil). Hydrobiologia 1997, 357, 89–98. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Barone, R. Importance of water-level fluctuation on population dynamics of cladocerans in a hypertrophic reservoir (Lake Arancio, south-west Sicily, Italy). Hydrobiologia 1997, 360, 223–232. [Google Scholar] [CrossRef]
- Heino, J.; Melo, A.S.; Siqueira, T.; Soininen, J.; Valanko, S.; Bini, L.M. Metacommunity organisation, spatial extent and dispersal in aquatic systems: Patterns, processes and prospects. Freshwat. Biol. 2015, 60, 845–869. [Google Scholar] [CrossRef]
- Reckendorfer, W.; Funk, A. Metacommunity structure in a floodplain system: Implications for conservation and restoration. In Proceedings of the 5th Symposium for Research in Protected Areas, Mittersill, Austria, 10–12 June 2013. [Google Scholar]
- Muneepeerakul, R.; Bertuzzo, E.; Lynch, H.J.; Fagan, W.F.; Rinaldo, A.; Rodriguez-Iturbe, I. Neutral metacommunity models predict fish diversity patterns in Mississippi-Missouri basin. Nature 2008, 453, 220-U229. [Google Scholar] [CrossRef] [Green Version]
- Cottenie, K.; Michels, E.; Nuytten, N.; De Meester, L. Zooplankton metacommunity structure: Regional vs. local processes in highly interconnected ponds. Ecology 2003, 84, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Urban, M.C. Disturbance heterogeneity determines freshwater metacommunity structure. Ecology 2004, 85, 2971–2978. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Song, K.; Pan, Y.; Wang, L.; Da, L.; Wang, Q. Metacommunity structure of zooplankton in river networks: Roles of environmental and spatial factors. Ecol. Indic. 2017, 73, 96–104. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Bini, L.M.; Bozelli, R.L. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 2007, 579, 1–13. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Funk, A.; Schiemer, F.; Reckendorfer, W. Metacommunity structure of aquatic gastropods in a river floodplain: The role of niche breadth and drift propensity. Freshwat. Biol. 2013, 58, 2505–2516. [Google Scholar] [CrossRef]
- Vanormelingen, P.; Cottenie, K.; Michels, E.; Muylaert, K.; Vyverman, W.; De Meester, L. The relative importance of dispersal and local processes in structuring phytoplankton communities in a set of highly interconnected ponds. Freshwat. Biol. 2008, 53, 2170–2183. [Google Scholar] [CrossRef]
- Dou, H.; Jiang, J. Dongting Lake; Press of University of Science and Technology of China: Hefei, China, 2000. [Google Scholar]
- Zhu, H.; Zhang, B. Poyang Lake; Press of University of Science and Technology of China: Hefei, China, 1997. [Google Scholar]
- Zhao, S.Q.; Fang, J.Y.; Miao, S.L.; Gu, B.; Tao, S.; Peng, C.H.; Tang, Z.Y. The 7-decade degradation of a large freshwater lake in central Yangtze river, China. Environ. Sci. Technol. 2005, 39, 431–436. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Zhang, Y.; Zhang, X.; Li, X. Enhanced lakebed sediment erosion in Dongting Lake induced by the operation of the Three Gorges Reservoir. J. Geogr. Sci. 2015, 25, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.L.; Zhang, Q.; Yao, J.; Werner, A.D.; Li, X.H. Hydrodynamic and Hydrological Modeling of the Poyang Lake Catchment System in China. J. Hydrol. Eng. 2014, 19, 607–616. [Google Scholar] [CrossRef]
- Błędzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Vieira, M.C.; Roitman, I.; Barbosa, H.d.O.; Machado Velho, L.F.; Galli Vieira, L.C. Spatial synchrony of zooplankton during the impoundment of amazonic reservoir. Ecol. Indic. 2019, 98, 649–656. [Google Scholar] [CrossRef]
- Brown, B.L.; Swan, C.M. Dendritic network structure constrains metacommunity properties in riverine ecosystems. J. Anim. Ecol. 2010, 79, 571–580. [Google Scholar] [CrossRef]
- Tavşanoğlu, Ü.N.; Šorf, M.; Stefanidis, K.; Brucet, S.; Türkan, S.; Agasild, H.; Baho, D.L.; Scharfenberger, U.; Hejzlar, J.; Papastergiadou, E. Effects of nutrient and water level changes on the composition and size structure of zooplankton communities in shallow lakes under different climatic conditions: A pan-European mesocosm experiment. Aquat. Ecol. 2017, 51, 257–273. [Google Scholar] [CrossRef]
- Tockner, K.; Malard, F.; Ward, J.V. An extension of the flood pulse concept. Hydrol. Processes 2000, 14, 2861–2883. [Google Scholar] [CrossRef]
- Mihaljevic, M.; Stevic, F.; Horvatic, J.; Kutuzovic, B.H. Dual impact of the flood pulses on the phytoplankton assemblages in a Danubian floodplain lake (Kopacki Rit Nature Park, Croatia). Hydrobiologia 2009, 618, 77–88. [Google Scholar] [CrossRef]
- Higuti, J.; Velho, L.F.M.; Lansac-Toha, F.A.; Martens, K. Pleuston communities are buffered from regional flood pulses: The example of ostracods in the Parana River floodplain, Brazil. Freshwat. Biol. 2007, 52, 1930–1943. [Google Scholar] [CrossRef]
- da Conceição, E.d.O.; Higuti, J.; de Campos, R.; Martens, K. Effects of flood pulses on persistence and variability of pleuston communities in a tropical floodplain lake. Hydrobiologia 2018, 807, 175–188. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ralph, T.J.; Ryder, D.S.; Hunter, S.J.; Shiel, R.J.; Segers, H. Spatial dissimilarities in plankton structure and function during flood pulses in a semi-arid floodplain wetland system. Hydrobiologia 2015, 747, 19–31. [Google Scholar] [CrossRef]
- Bozelli, R.L.; Thomaz, S.M.; Padial, A.A.; Lopes, P.M.; Bini, L.M. Floods decrease zooplankton beta diversity and environmental heterogeneity in an Amazonian floodplain system. Hydrobiologia 2015, 753, 233–241. [Google Scholar] [CrossRef]
- Braghin, L.S.M.; Figueiredo, B.R.S.; Meurer, T.; Michelan, T.S.; Simoes, N.R.; Bonecker, C.C. Zooplankton diversity in a dammed river basin is maintained by preserved tributaries in a tropical floodplain. Aquat. Ecol. 2015, 49, 175–187. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Jeppesen, E.; Chen, Y.; Liu, X.; Zhang, W. Horizontal distribution of pelagic crustacean zooplankton biomass and body size in contrasting habitat types in Lake Poyang, China. Environ. Sci. Pollut. Res. 2019, 26, 2270–2280. [Google Scholar] [CrossRef]
- Kraus, C.N.; Bonnet, M.-P.; Miranda, C.A.; Nogueira, I.d.S.; Garnier, J.; Galli Vieira, L.C. Interannual hydrological variations and ecological phytoplankton patterns in Amazonian floodplain lakes. Hydrobiologia 2019, 830, 135–149. [Google Scholar] [CrossRef] [Green Version]
- Chaparro, G.; Horvath, Z.; O’Farrell, I.; Ptacnik, R.; Hein, T. Plankton metacommunities in floodplain wetlands under contrasting hydrological conditions. Freshwat. Biol. 2018, 63, 380–391. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.D.; Simoes, N.R.; Meerhoff, M.; Lansac-Toha, F.A.; Machado Velho, L.F.; Bonecker, C.C. Hydrological dynamics drives zooplankton metacommunity structure in a Neotropical floodplain. Hydrobiologia 2016, 781, 109–125. [Google Scholar] [CrossRef]
- Strecker, A.L.; Cobb, T.P.; Vinebrooke, R.D. Effects of experimental greenhouse warming on phytoplankton and zooplankton communities in fishless alpine ponds. Limnol. Oceanogr. 2004, 49, 1182–1190. [Google Scholar] [CrossRef]
- Havens, K.E.; Beaver, J.R. Zooplankton to phytoplankton biomass ratios in shallow Florida lakes: An evaluation of seasonality and hypotheses about factors controlling variability. Hydrobiologia 2013, 703, 177–187. [Google Scholar] [CrossRef]
- Havens, K.E.; Beaver, J.R.; Manis, E.E.; East, T.L. Inter-lake comparisons indicate that fish predation, rather than high temperature, is the major driver of summer decline in Daphnia and other changes among cladoceran zooplankton in subtropical Florida lakes. Hydrobiologia 2015, 750, 57–67. [Google Scholar] [CrossRef]
- Wang, J.; Shen, J.; Wu, Y.; Tu, C.; Soininen, J.; Stegen, J.C.; He, J.; Liu, X.; Zhang, L.; Zhang, E. Phylogenetic beta diversity in bacterial assemblages across ecosystems: Deterministic versus stochastic processes. ISME J. 2013, 7, 1310–1321. [Google Scholar] [CrossRef]
- Chase, J.M.; Myers, J.A. Disentangling the importance of ecological niches from stochastic processes across scales. Philos. Trans. R. Soc. B-Biol. Sci. 2011, 366, 2351–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Li, Y.-l.; Liu, B.-G.; Qian, K.-M.; Chen, Y.-W.; Gao, J.-F. Cyanobacteria in the complex river-connected Poyang Lake: Horizontal distribution and transport. Hydrobiologia 2016, 768, 95–110. [Google Scholar] [CrossRef]
- Humphries, S. Filter feeders and plankton increase particle encounter rates through flow regime control. Proc. Natl. Acad. Sci. USA 2009, 106, 7882–7887. [Google Scholar] [CrossRef] [Green Version]
- Pinel-Alloul, B.; Andre, A.; Legendre, P.; Cardille, J.A.; Patalas, K.; Salki, A. Large-scale geographic patterns of diversity and community structure of pelagic crustacean zooplankton in Canadian lakes. Glob. Ecol. Biogeogr. 2013, 22, 784–795. [Google Scholar] [CrossRef]
- Otto, S.A.; Diekmann, R.; Flinkman, J.; Kornilovs, G.; Mollmann, C. Habitat Heterogeneity Determines Climate Impact on Zooplankton Community Structure and Dynamics. PLoS ONE 2014, 9, e90875. [Google Scholar] [CrossRef] [Green Version]
- Vijverberg, J.; Boersma, M. Long-term dynamics of small-bodied and large-bodied cladocerans during the eutrophication of a shallow reservoir, with special attention for Chydorus sphaericus. Hydrobiologia 1997, 360, 233–242. [Google Scholar] [CrossRef]
- Thorp, J.H.; Mantovani, S. Zooplankton of turbid and hydrologically dynamic prairie rivers. Freshwat. Biol. 2005, 50, 1474–1491. [Google Scholar] [CrossRef]
- Wahl, D.H.; Goodrich, J.; Nannini, M.A.; Dettmers, J.M.; Soluk, D.A. Exploring riverine zooplankton in three habitats of the Illinois River ecosystem: Where do they come from? Limnol. Oceanogr. 2008, 53, 2583–2593. [Google Scholar] [CrossRef] [Green Version]
- Kong, X.; He, Q.; Yang, B.; He, W.; Xu, F.; Janssen, A.B.G.; Kuiper, J.J.; van Gerven, L.P.A.; Qin, N.; Jiang, Y.; et al. Hydrological regulation drives regime shifts: Evidence from paleolimnology and ecosystem modeling of a large shallow Chinese lake. Glob. Chang. Biol. 2017, 23, 737–754. [Google Scholar] [CrossRef]
- Yin, H.F.; Li, C.G. Human impact on floods and flood disasters on the Yangtze River. Geomorphology 2001, 41, 105–109. [Google Scholar] [CrossRef]
- Lougheed, V.L.; McIntosh, M.D.; Parker, C.A.; Stevenson, R.J. Wetland degradation leads to homogenization of the biota at local and landscape scales. Freshwat. Biol. 2008, 53, 2402–2413. [Google Scholar] [CrossRef]
- Obolewski, K.; Glińska-Lewczuk, K.; Ożgo, M.; Astel, A. Connectivity restoration of floodplain lakes: An assessment based on macroinvertebrate communities. Hydrobiologia 2016, 774, 23–37. [Google Scholar] [CrossRef] [Green Version]
- Vellend, M.; Verheyen, K.; Flinn, K.M.; Jacquemyn, H.; Kolb, A.; Van Calster, H.; Peterken, G.; Graae, B.J.; Bellemare, J.; Honnay, O.; et al. Homogenization of forest plant communities and weakening of species-environment relationships via agricultural land use. J. Ecol. 2007, 95, 565–573. [Google Scholar] [CrossRef]
- Monchamp, M.-E.; Spaak, P.; Domaizon, I.; Dubois, N.; Bouffard, D.; Pomati, F. Homogenization of lake cyanobacterial communities over a century of climate change and eutrophication. Nat. Ecol. Evol. 2018, 2, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Smart, S.M.; Thompson, K.; Marrs, R.H.; Le Duc, M.G.; Maskell, L.C.; Firbank, L.G. Biotic homogenization and changes in species diversity across human-modified ecosystems. Proc. R. Soc. B-Biol. Sci. 2006, 273, 2659–2665. [Google Scholar] [CrossRef] [Green Version]
- Olden, J.D.; Comte, L.; Giam, X. The Homogocene: A research prospectus for the study of biotic homogenisation. Neobiota 2018, 37, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; McGowan, S.; Cao, Y.; Chen, X. Effects of dam construction and increasing pollutants on the ecohydrological evolution of a shallow freshwater lake in the Yangtze floodplain. Sci. Total Environ. 2018, 621, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Shankman, D.; Keim, B.D.; Song, J. Flood frequency in China’s Poyang Lake region: Trends and teleconnections. Int. J. Climatol. 2006, 26, 1255–1266. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, D.L.; Gigney, H.; Watson, G. Riverine habitat heterogeneity: The role of slackwaters in providing hydrologic buffers for benthic microfauna. Hydrobiologia 2010, 638, 181–191. [Google Scholar] [CrossRef]
- Liu, B.; Tan, G.; Xing, J.; Li, M.; Chen, Y. Effect of Pen Culture on Community Structure of Planktonic Crustaceans in Lake Junshan. J. Ecol. Rural Environ. 2015, 31, 82–87. [Google Scholar]
- Zhou, S.C.; Tang, T.; Wu, N.C.; Fu, X.C.; Cai, Q.H. Impacts of a small dam on riverine zooplankton. Int. Rev. Hydrobiol. 2008, 93, 297–311. [Google Scholar] [CrossRef]
- Bortolini, J.C.; Pineda, A.; Rodrigues, L.C.; Jati, S.; Velho, L.F.M. Environmental and spatial processes influencing phytoplankton biomass along a reservoirs–river–floodplain lakes gradient: A metacommunity approach. Freshwat. Biol. 2017, 62, 1756–1767. [Google Scholar] [CrossRef]
- Langenheder, S.; Berga, M.; Östman, Ö.; Székely, A.J. Temporal variation of β-diversity and assembly mechanisms in a bacterial metacommunity. ISME J. 2012, 6, 1107–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Xu, H.; Vilmi, A.; Tolonen, K.T.; Tang, X.; Qin, B.; Gong, Z.; Heino, J. Relative roles of spatial processes, natural factors and anthropogenic stressors in structuring a lake macroinvertebrate metacommunity. Sci. Total Environ. 2017, 601, 1702–1711. [Google Scholar] [CrossRef]
- de Melo, M.L.; Bertilsson, S.; Amaral, J.H.F.; Barbosa, P.M.; Forsberg, B.R.; Sarmento, H. Flood pulse regulation of bacterioplankton community composition in an Amazonian floodplain lake. Freshwat. Biol. 2019, 64, 108–120. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhou, C.; Zheng, L.; Duan, H.; Chen, Y.; Wang, G. Metacommunity Concepts Provide New Insights in Explaining Zooplankton Spatial Patterns within Large Floodplain Systems. Water 2022, 14, 93. https://doi.org/10.3390/w14010093
Liu B, Zhou C, Zheng L, Duan H, Chen Y, Wang G. Metacommunity Concepts Provide New Insights in Explaining Zooplankton Spatial Patterns within Large Floodplain Systems. Water. 2022; 14(1):93. https://doi.org/10.3390/w14010093
Chicago/Turabian StyleLiu, Baogui, Chuanqiao Zhou, Lilin Zheng, Haixin Duan, Ying Chen, and Guoxiang Wang. 2022. "Metacommunity Concepts Provide New Insights in Explaining Zooplankton Spatial Patterns within Large Floodplain Systems" Water 14, no. 1: 93. https://doi.org/10.3390/w14010093