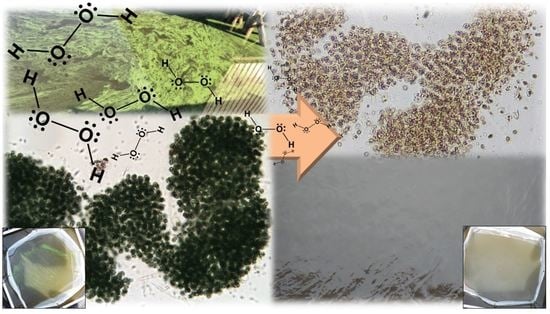
Evaluation of a Peroxide-Based Algaecide for Cyanobacteria Control: A Mesocosm Trial in Lake Okeechobee, FL, USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Study Design
2.2. Sample Collection
2.3. In Situ Measurement Methods
2.4. Peroxide Monitoring
2.5. Extracted Chlorophyll
2.6. Molecular Methods
2.7. Microscopic Identification and Enumeration
2.8. Total Toxin
2.9. Statistical Analyses
3. Results
3.1. Peroxide Concentrations
3.2. Water Quality
3.3. Biomass Assessment
3.3.1. Phytoplankton
3.3.2. Cyanobacteria
3.4. Community Level Changes
3.5. Cyanotoxins
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeppesen, E.; Søndergaard, M.; Meerhoff, M.; Lauridsen, T.; Jensen, J.P. Shallow lake restoration by nutrient loading reduction—some recent findings and challenges ahead. Hydrobiologia 2007, 584, 239–252. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; Hall, N.S.; Zhu, G.; Qin, B.; Wu, Y.; Rossignol, K.L.; Dong, L.; McCarthy, M.J.; Joyner, A.R. Controlling Cyanobacterial Blooms in Hypertrophic Lake Taihu, China: Will Nitrogen Reductions Cause Replacement of Non-N2 Fixing by N2 Fixing Taxa? PLoS ONE 2014, 9, e113123. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.A.; Rollwagen-Bollens, G.; Bollens, S.M.; Faber-Hammond, J.J. Environmental influence on cyanobacteria abundance and microcystin toxin production in a shallow temperate lake. Ecotoxicol. Environ. Saf. 2015, 114, 318–325. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms Like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Dziallas, C.; Grossart, H.P. Increasing oxygen radicals and water temperature select for toxic Microcystis sp. PLoS ONE 2011, 6, e255869. [Google Scholar] [CrossRef] [Green Version]
- Almanza, V.; Pedreros, P.; Laughinghouse IV, H.D.; Félez, J.; Parra, O.; Azócar, M.; Urrutia, R. Association between trophic state, watershed use, and blooms of cyanobacteria in south-central Chile. Limnologica 2019, 75, 30–41. [Google Scholar] [CrossRef]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.; Johnson, M.V.V.; Morton, S.L.; Perkins, D.A.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Boyer, G.L. Health impacts from cyanobacteria harmful algae blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Gardner, W.S.; Havens, K.E.; Joyner, A.R.; McCarthy, M.J.; Newell, S.; Qin, B.; Scott, J.T. Mitigating cyanobacterial harmful algal blooms in aquatic ecosystems impacted by climate change and anthropogenic nutrients. Harmful Algae 2016, 54, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthijs, H.C.; Visser, P.M.; Reeze, B.; Meeuse, J.; Slot, P.C.; Wijn, G.; Talens, R.; Huisman, J. Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res. 2012, 46, 1460–1472. [Google Scholar] [CrossRef]
- Geer, T.D.; Calomeni, A.J.; Kinley, C.M.; Iwinski, K.J.; Rodgers, J.H. Predicting In Situ Responses of Taste- and Odor-Producing Algae in a Southeastern US Reservoir to a Sodium Carbonate Peroxyhydrate Algaecide Using a Laboratory Exposure-Response Model. Water Air Soil Pollut. 2017, 228, 1–14. [Google Scholar] [CrossRef]
- Laughinghouse, H.D., IV; Berthold, D.E.; Bishop, W.M. Approaches to managing cyanobacterial blooms and altering water quality. Aquatics 2020, 42, 13–16. [Google Scholar]
- Kinley-Baird, C.; Calomeni, A.J.; Berthold, D.E.; Lefler, F.W.; Barbosa, M.; Rodgers, J.H.; Laughinghouse IV, H.D. Laboratory-scale evaluation of algaecide effectiveness for control of microcystin-producing cyanobacteria from Lake Okeechobee, Florida (USA). Ecotoxicol. Environ. Saf. 2021, 207, 111233. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.A.; Guedes, D.O.; Barros, M.U.; Oliveira, S.; Pacheco, A.B.; Azevedo, S.M.; Magalhães, V.F.; Pestana, C.J.; Edwards, C.; Lawton, L.A.; et al. Effect of hydrogen peroxide on natural phytoplankton and bacterioplankton in a drinking water reservoir: Mesocosm-scale study. Water Res. 2021, 197, 117069. [Google Scholar] [CrossRef]
- Reichwaldt, E.S.; Zheng, L.; Barrington, D.J.; Ghadouani, A. Acute Toxicological Response of Daphnia and Moina to Hydrogen Peroxide. J. Environ. Eng. 2012, 138, 607–611. [Google Scholar] [CrossRef] [Green Version]
- Barrington, D.; Reichwaldt, E.; Ghadouani, A. The use of hydrogen peroxide to remove cyanobacteria and microcystins from waste stabilization ponds and hypereutrophic systems. Ecol. Eng. 2013, 50, 86–94. [Google Scholar] [CrossRef]
- Weenink, E.F.J.; Luimstra, V.M.; Schuurmans, J.M.; Van Herk, M.J.; Visser, P.M.; Matthijs, H.C.P. Combatting cyanobacteria with hydrogen peroxide: A laboratory study on the consequences for phytoplankton community and diversity. Front. Microbiol. 2015, 6, 714. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Buley, R.P.; Fernandez-Figueroa, E.G.; Barros, M.U.; Rajendran, S.; Wilson, A.E. Hydrogen peroxide treatment promotes chlorophytes over toxic cyanobacteria in a hyper-eutrophic aquaculture pond. Environ. Pollut. 2018, 240, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Song, Q.; Long, J.; Song, G.; Mi, W.; Bi, Y. Optimization method for Microcystis bloom mitigation by hydrogen peroxide and its stimulative effects on growth of chlorophytes. Chemosphere 2019, 228, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, L.; Aguilera, A.; Echenique, R.; Andrinolo, D.; Giannuzzi, L. Application of Hydrogen Peroxide to the Control of Eutrophic Lake Systems in Laboratory Assays. Toxins 2014, 6, 2657–2675. [Google Scholar] [CrossRef]
- SePRO. PAK 27 Algaecide Product Label; SePRO Corporation: Carmel, IN, USA, 2018. [Google Scholar]
- Sinha, A.K.; Eggleton, M.A.; Lochmann, R.T. An environmentally friendly approach for mitigating cyanobacteria bloom and their toxins in hypereutrophic ponds: Potentiality of a newly developed granular hydrogen peroxide based compound. Sci. Total Environ. 2018, 637–638, 524–537. [Google Scholar] [CrossRef]
- Cornish, B.J.P.A.; Lawton, L.A.; Robertson, P.K.J. Hydrogen peroxide enhanced photocatalytic oxidation of microcystin-LR using titanium dioxide. Appl. Catal. B-Environ. 2000, 25, 59–67. [Google Scholar] [CrossRef]
- Bandala, E.R.; Martínez, D.; Martínez, E.; Dionysiou, D.D. Degradation of microcystin-LR toxin by Fenton and Photo-Fenton processes. Toxicon 2004, 43, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Sorlini, S.; Collivignarelli, C.; Miino, M.C.; Caccamo, F.M.; Collivignarelli, M.C. Kinetics of Microcystin-LR Removal in a Real Lake Water by UV/H2O2 Treatment and Analysis of Specific Energy Consumption. Toxins 2020, 12, 810. [Google Scholar] [CrossRef] [PubMed]
- Piel, T.; Sandrini, G.; White, E.; Xu, T.; Schuurmans, J.M.; Huisman, J.; Visser, P.M. Suppressing Cyanobacteria with Hydrogen Peroxide Is More Effective at High Light Intensities. Toxins 2019, 12, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandrini, G.; Piel, T.; Xu, T.S.; White, E.; Qin, H.J.; Slot, P.C.; Huisman, J.; Visser, P.M. Sensitivity to hydrogen peroxide of the bloom-forming cyanobacterium Microcystis PCC 7806 depends on nutrient availability. Harmful Algae 2020, 99, 101916. [Google Scholar] [CrossRef] [PubMed]
- Mitman, G.G.; Tucci, N. Mine Waste Technology Program Activity IV, Project 30 Final Report-Algal Bioremediation of the Berkeley Pit lake System: An In Situ Test Using Limnocorrals; EPA publications MWTP-275: Cincinnati, OH, USA, 2007; 52p. [Google Scholar]
- Klassen, N.V.; Marchington, D.; McGowan, H.C.E. Hydrogen peroxide determination by the I3- method and by KMnO4 titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Kinley, C.M.; Rodgers, J.H.; Iwinski, K.J.; McQueen, A.D.; Calomeni, A.J. Analysis of Algaecide Exposures: An Evaluation of the I3 − Method to Measure Sodium Carbonate Peroxyhydrate Algaecides. Water Air Soil Pollut. 2015, 226, 170. [Google Scholar] [CrossRef]
- Arar, E.J. Method 4460.0: In Vitro Determination of Chlorophylls a, b, c + c and Pheopigments in 12 Marine and Freshwater Algae by Visible Spectrophotometry; U.S. Environmental Protection Agency: Washington, DC, USA, 1997.
- Chiu, Y.T.; Chen, Y.H.; Wang, T.S.; Yen, H.K.; Lin, T.F. A qPCR-based tool to diagnose the presence of harmful cyanobacteria and cyanotoxins in drinking water sources. Int. J. Environ. Res. Public Health 2017, 14, 547. [Google Scholar] [CrossRef] [Green Version]
- Lund, J.W.G.; Kipling, C.; Le Cren, E.D. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 1958, 11, 143–170. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Pappas, J.L.; Stoermer, E.F. Quantitative method for determining a representative algal sample count1. J. Phycol. 1996, 32, 693–696. [Google Scholar] [CrossRef] [Green Version]
- Bicudo, C.E.M.; Mezes, M. Gêneors de algas de águas continentais do Brasil: Chave de identificação e descrições; RiMa Editora: São Carlos, Brazil, 2005; 508p. [Google Scholar]
- Cox, E.J. Identification of Freshwater Diatoms from Live Material; Chapman and Hall: London, UK, 1996; p. 158. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 1. Chroococcales. In Süßwasserflora von Mitteleuropa Teil 19/1; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer, Jena-StuttgartLübeck-Ulm: Heidelberg, Germany, 1998; p. 548. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 2. Oscillatoriales. In Süßwasserflora von Mitteleuropa Teil 19/2; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier/Spektrum: Heidelberg, Germany, 2005; p. 759. [Google Scholar]
- Komárek, J. Cyanoprokaryota. 3. Heterocytous genera. In Süßwasserflora von Mitteleuropa Teil 19/3; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer Spektrum: Berlin, Heidelberg, Germany, 2013; p. 1130. [Google Scholar]
- Wehr, J.D.; Sheath, R.G.; Kociolek, J.P. (Eds.) Freshwater Algae of North America, 2nd ed; Academic Press: Amsterdam, The Netherlands, 2015; 1050p. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 2020. Available online: http://www.algaebase.org (accessed on 10 December 2020).
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association; American Water Works Association; Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Zaffiro, A.; Rosenblum, L.; Wendelken, S.C. Method 546: Determination of Total Microcystis and Nodularins in Drinking Water and Ambient Water by Adda Enzyme-Linked Immunosorbent Assay; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2016.
- Medina, V.F.; Pokrzywinski, K.L.; Martinez-Guerra, E.L. Evaluation of Koontz Lake (North Indiana) Ecological Restoration Options–Comparison of Dredging and Aeration–and Broad Application to USACE Projects; USACE Technical Report, ERDC/EL TR-18-2; Environmental Laboratory, Engineer Research and Development Center: Vicksburg, MS, USA, 2018. [Google Scholar]
- Ohio Watershed Network. Specific Conductivity. Ohio State University Extension. 2019. Available online: https://ohiowatersheds.osu.edu/node/1493 (accessed on 4 April 2021).
- Chorus, I.; Bartram, J. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; World Health Organization. E & FN Spon: New York, NY, USA, 1999; 446p. [Google Scholar]
- Bishop, W.M.; Johnson, B.M.; Rodgers, J.H., Jr. Comparative responses of target and non-target species to exposures of a copper-based algaecide. J. Aquat. Plant Manage. 2014, 52, 65–70. [Google Scholar]
- Closson, K.R.; Paul, E.A. Comparison of the Toxicity of Two Chelated Copper Algaecides and Copper Sulfate to Non-Target Fish. Bull. Environ. Contam. Toxicol. 2014, 93, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Gaikowski, M.P.; Rach, J.J.; Ramsay, R.T. Acute toxicity of hydrogen peroxide treatments to selected life stages of cold-, cool-, and warmwater fish. Aquaculture 1999, 178, 191–207. [Google Scholar] [CrossRef]
- Geer, T.D.; Kinley, C.M.; Iwinski, K.J.; Calomeni, A.J.; Rodgers, J.H. Comparative toxicity of sodium carbonate peroxyhydrate to freshwater organisms. Ecotoxicol. Environ. Saf. 2016, 132, 202–211. [Google Scholar] [CrossRef]
- Drábková, M.; Admiraal, W.; Maršálek, B. Combined Exposure to Hydrogen Peroxide and LightSelective Effects on Cyanobacteria, Green Algae, and Diatoms. Environ. Sci. Technol. 2007, 41, 309–314. [Google Scholar] [CrossRef]
- Drábková, M.; Matthijs, H.C.P.; Admiraal, W.; Marsalek, B. Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 2007, 45, 363–369. [Google Scholar] [CrossRef]
- Matthijs, H.C.P.; Jančula, D.; Visser, P.M.; Maršálek, B. Existing and emerging cyanocidal compounds: New perspectives for cyanobacterial bloom mitigation. Aquat. Ecol. 2016, 50, 443–460. [Google Scholar] [CrossRef] [Green Version]
- Latifi, A.; Ruiz, M.; Zhang, C.-C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, W.T.; Yerby, J.N.; Carlee, J.; Bishop, W.M.; Willis, B.E.; Horton, C.T. Controlling Lyngbya wollei in three Alabama, USA reservoirs: Summary of a long-term management program. Appl. Water Sci. 2019, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Cottingham, K.; Ewing, H.A.; Greer, M.; Carey, C.C.; Weathers, K.C. Cyanobacteria as biological drivers of lake nitrogen and phosphorus cycling. Ecosphere 2015, 6, art1. [Google Scholar] [CrossRef]
- Osman, O.A.; Beier, S.; Grabherr, M.; Bertilsson, S. Interactions of Freshwater Cyanobacteria with Bacterial Antagonists. Appl. Environ. Microbiol. 2017, 83, e02634-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Li, L.; Huang, L.; Guo, L.; Song, L. Combining hydrogen peroxide addition with sunlight regulation to control algal blooms. Environ. Sci. Pollut. Res. 2018, 25, 2239–2247. [Google Scholar] [CrossRef] [PubMed]







| Diversity (H′) | Evenness (J′) | |||||
|---|---|---|---|---|---|---|
| Time | Untreated | Single | Sequential | Untreated | Single | Sequential |
| Pretreatment | 2.270 | 2.386 | 2.415 | 0.8150 | 0.8510 | 0.8425 |
| 24 h | 2.528 | 2.464 | NA | 0.8311 | 0.8473 | NA |
| 72 h | 2.401 | 2.406 | 2.457 | 0.8088 | 0.8179 | 0.8446 |
| NA indicates no analysis was conducted | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokrzywinski, K.L.; Bishop, W.M.; Grasso, C.R.; Fernando, B.M.; Sperry, B.P.; Berthold, D.E.; Laughinghouse, H.D., IV; Van Goethem, E.M.; Volk, K.; Heilman, M.; et al. Evaluation of a Peroxide-Based Algaecide for Cyanobacteria Control: A Mesocosm Trial in Lake Okeechobee, FL, USA. Water 2022, 14, 169. https://doi.org/10.3390/w14020169
Pokrzywinski KL, Bishop WM, Grasso CR, Fernando BM, Sperry BP, Berthold DE, Laughinghouse HD IV, Van Goethem EM, Volk K, Heilman M, et al. Evaluation of a Peroxide-Based Algaecide for Cyanobacteria Control: A Mesocosm Trial in Lake Okeechobee, FL, USA. Water. 2022; 14(2):169. https://doi.org/10.3390/w14020169
Chicago/Turabian StylePokrzywinski, Kaytee L., West M. Bishop, Christopher R. Grasso, Brianna M. Fernando, Benjamen P. Sperry, David E. Berthold, Haywood Dail Laughinghouse, IV, Erika M. Van Goethem, Kaitlin Volk, Mark Heilman, and et al. 2022. "Evaluation of a Peroxide-Based Algaecide for Cyanobacteria Control: A Mesocosm Trial in Lake Okeechobee, FL, USA" Water 14, no. 2: 169. https://doi.org/10.3390/w14020169