Enhanced Photo-Fenton Activity Using Magnetic Cu0.5Mn0.5Fe2O4 Nanoparticles as a Recoverable Catalyst for Degrading Organic Contaminants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalysts
2.2. Photo-Fenton Catalytic Performance Evaluation
2.3. Material Adsorption Capacity and pH of Zero Point of Charge (pHpzc)
2.4. Quenching Experiments
2.5. Recycling Experiments
2.6. Effect of Real Water
2.7. Pilot-Scale Experiment
2.8. Toxicological Evaluation
3. Results and Discussion
3.1. Material Characterization
3.2. Degradation Performance
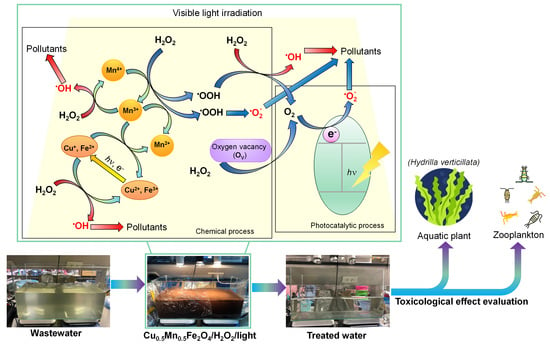
3.3. Photo-Fenton Mechanism
3.4. Reusability
3.5. Effect of Real Wastewater
3.6. Pilot-Scale Experiment and Toxicological Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.M. Degradation and mineralization, of organic contaminants by Fenton and photo-Fenton processes: Review of mechanisms and effects of organic and inorganic additives. Res. J. Chem. Environ. 2011, 15, 96–112. [Google Scholar]
- Yang, Y.; Ok, Y.S.; Kim, K.-H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total Environ. 2017, 596–597, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Kalantary, R.; Barzegar, G.; Jorfi, S. Monitoring of pesticides in surface water, pesticides removal efficiency in drinking water treatment plant and potential health risk to consumers using Monte Carlo simulation in Behbahan City, Iran. Chemosphere 2022, 286, 131667. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Kong, L.; Jing, Z.; Wang, S.; Lai, Y.; Xie, M.; Ma, L.; Feng, Z.; Zhan, J. Facile synthesis of superparamagnetic β-CD-MnFe2O4 as a peroxymonosulfate activator for efficient removal of 2,4- dichlorophenol: Structure, performance, and mechanism. J. Hazard. Mater. 2020, 394, 122528. [Google Scholar] [CrossRef]
- Wang, X.; Wang, A.; Ma, J. Visible-light-driven photocatalytic removal of antibiotics by newly designed C3N4@MnFe2O4-graphene nanocomposites. J. Hazard. Mater. 2017, 336, 81–92. [Google Scholar] [CrossRef]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S. Fabrication of novel magnetic MnFe2O4/bio-char composite and heterogeneous photo-Fenton degradation of tetracycline in near neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef]
- Peng, W.; Zhang, K.; Zong, F.; Chen, C.; Fang, Z. Enhancement of H2O2 decomposition by the synergistic effect on CuO-MnFe2O4 nanoparticles for sulfamethoxazole degradation over a wide pH range. J. Dispers. Sci. Technol. 2020, 41, 2211–2222. [Google Scholar] [CrossRef]
- Wang, F.-X.; Wang, C.-C.; Du, X.; Li, Y.; Wang, F.; Wang, P. Efficient removal of emerging organic contaminants via photo-Fenton process over micron-sized Fe-MOF sheet. Chem. Eng. J. 2022, 429, 132495. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Qin, H.; Zhou, J.; Shen, Q.; Wang, K.; Chen, W.; Liu, M.; Li, N. Synergy effect between adsorption and heterogeneous photo-Fenton-like catalysis on LaFeO3/lignin-biochar composites for high efficiency degradation of ofloxacin under visible light. Sep. Purif. Technol. 2022, 280, 119751. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wang, S.; Yu, P.; Xu, Y.; Sun, Y. Highly enhanced heterogeneous photo-Fenton process for tetracycline degradation by Fe/SCN Fenton-like catalyst. J. Environ. Manag. 2022, 312, 114856. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Zhang, C.; Feng, J.; Pu, S.; Ren, Y.; Wang, Y. Enhanced catalytic ozonation treatment of dibutyl phthalate enabled by porous magnetic Ag-doped ferrospinel MnFe2O4 materials: Performance and mechanism. Chem. Eng. J. 2018, 354, 42–52. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, C.; Qin, L.; Fu, Y.; He, J.; Huang, D.; Li, B.; Zhang, M.; Liu, S.; Li, L.; et al. ZIF-8-modified MnFe2O4 with high crystallinity and superior photo-Fenton catalytic activity by Zn-O-Fe structure for TC degradation. Chem. Eng. J. 2020, 392, 124851. [Google Scholar] [CrossRef]
- Angkaew, A.; Chokejaroenrat, C.; Sakulthaew, C.; Mao, J.; Watcharatharapong, T.; Watcharenwong, A.; Imman, S.; Suriyachai, N.; Kreetachat, T. Two facile synthesis routes for magnetic recoverable MnFe2O4/g-C3N4 nanocomposites to enhance visible light photo-Fenton activity for methylene blue degradation. J. Environ. Chem. Eng. 2021, 9, 105621. [Google Scholar] [CrossRef]
- Huang, G.-X.; Wang, C.-Y.; Yang, C.-W.; Guo, P.-C.; Yu, H.-Q. Degradation of Bisphenol A by Peroxymonosulfate Catalytically Activated with Mn1.8Fe1.2O4 Nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Liu, X.; Jiang, X.; Zhang, Z.; Zhang, T.; Zhang, L. The investigation of synergistic and competitive interaction between dye Congo red and methyl blue on magnetic MnFe2O4. Chem. Eng. J. 2014, 246, 88–96. [Google Scholar] [CrossRef]
- Velinov, N.; Petrova, T.; Tsoncheva, T.; Genova, I.; Koleva, K.; Kovacheva, D.; Mitov, I. Auto-combustion synthesis, Mössbauer study and catalytic properties of copper-manganese ferrites. Hyperfine Interact. 2016, 237, 1–11. [Google Scholar] [CrossRef]
- Zhan, Y.; Meng, Y.; Li, W.; Chen, Z.; Yan, N.; Li, Y.; Teng, M. Magnetic recoverable MnFe2O4/cellulose nanocrystal composites as an efficient catalyst for decomposition of methylene blue. Ind. Crops Prod. 2018, 122, 422–429. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, Z.; Zhang, X.; Ding, M.; Huang, S.; Yang, S. Magnetic recyclable MnFe2O4/CeO2/SnS2 ternary nano-photocatalyst for photo-Fenton degradation. Appl. Catal. A Gen. 2020, 593, 117443. [Google Scholar] [CrossRef]
- Rana, M.U.; Misbah-ul-Islam; Abbas, T. Magnetic interactions in Cu-substituted manganese ferrites. Solid State Commun. 2003, 126, 129–133. [Google Scholar] [CrossRef]
- Wu, K.; Wang, M.; Li, A.; Zhao, Z.; Liu, T.; Hao, X.; Yang, S.; Jin, P. The enhanced As(III) removal by Fe-Mn-Cu ternary oxide via synergistic oxidation: Performances and mechanisms. Chem. Eng. J. 2021, 406, 126739. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, J.; Liu, D.; Li, X.; Liang, H. Enhanced catalytic performance of Cu-doped MnFe2O4 magnetic ferrites: Tetracycline hydrochloride attacked by superoxide radicals efficiently in a strong alkaline environment. Chemosphere 2022, 297, 134154. [Google Scholar] [CrossRef]
- Meena, S.; Renuka, L.; Anantharaju, K.S.; Vidya, Y.S.; Nagaswarupa, H.P.; Prashantha, S.C.; Nagabhushana, H. Optical, Electrochemical and Photocatalytic Properties of Sunlight Driven Cu Doped Manganese Ferrite Synthesized by Solution Combustion Synthesis. Mater. Today Proc. 2017, 4, 11773–11781. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res. 2004, 38, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Tadic, M.; Panjan, M.; Damnjanovic, V.; Milosevic, I. Magnetic properties of hematite (α-Fe2O3) nanoparticles prepared by hydrothermal synthesis method. Appl. Surf. Sci. 2014, 320, 183–187. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Zeng, D.; Zhang, B.; Hassan, M.; Li, P.; Qi, C.; He, Y. Enhanced catalytic activation of photo-Fenton process by Cu0·5Mn0·5Fe2O4 for effective removal of organic contaminants. Chemosphere 2020, 247, 125780. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Z.; Liu, J.; Shan, N.; Zhang, H.; Dionysiou, D.D. Visible light-assisted heterogeneous Fenton with ZnFe2O4 for the degradation of Orange II in water. Appl. Catal. B Environ. 2016, 182, 456–468. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, Z.; Li, Y.; Liu, Y.; Chen, Y.; Wu, Y.; Zhang, J.; Li, H.; Xu, R.; Wang, S.; et al. Glucose enhanced the oxidation performance of iron-manganese binary oxides: Structure and mechanism of removing tetracycline. J. Colloid Interface Sci. 2020, 573, 287–298. [Google Scholar] [CrossRef]
- Cheng, D.; Yan, C.; Liu, Y.; Zhou, Y.; Lu, D.; Tang, X.; Cai, G.; Li, D.; Zhao, Z.; Wang, X. Loading CuFe2O4 onto ceramic fabric for photocatalytic degradation of methylene blue under visible light irradiation. Ceram. Int. 2022, 48, 1256–1263. [Google Scholar] [CrossRef]
- Utset, B.; Garcia, J.; Casado, J.; Domènech, X.; Peral, J. Replacement of H2O2 by O2 in Fenton and photo-Fenton reactions. Chemosphere 2000, 41, 1187–1192. [Google Scholar] [CrossRef]
- Hashemian, S.; Mirshamsi, M. Kinetic and thermodynamic of adsorption of 2-picoline by sawdust from aqueous solution. J. Ind. Eng. Chem. 2012, 18, 2010–2015. [Google Scholar] [CrossRef]
- Punamiya, P.; Sarkar, D.; Rakshit, S.; Datta, R. Effectiveness of Aluminum-based Drinking Water Treatment Residuals as a Novel Sorbent to Remove Tetracyclines from Aqueous Medium. J. Environ. Qual. 2013, 42, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently Enhancing Electrocatalytic Activity of α-MnO2 Nanorods/N-Doped Ketjenblack Carbon for Oxygen Reduction Reaction and Oxygen Evolution Reaction Using Facile Regulated Hydrothermal Treatment. Catalysts 2018, 8, 138. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xiao, B.; Liu, S.-Q.; Meng, Z.; Chen, Z.-G.; Zou, C.-Y.; Liu, C.-B.; Chen, F.; Zhou, X. Photo-Fenton degradation of ammonia via a manganese–iron double-active component catalyst of graphene–manganese ferrite under visible light. Chem. Eng. J. 2016, 283, 266–275. [Google Scholar] [CrossRef]
- Oberländer, J.; Kirchner, P.; Boyen, H.-G.; Schöning, M.J. Detection of hydrogen peroxide vapor by use of manganese(IV) oxide as catalyst for calorimetric gas sensors. Phys. Status Solidi A 2014, 211, 1372–1376. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Zhao, H.; Cao, T.; Qian, L.; Wang, Y.; Zhao, G. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J. Mol. Catal. A Chem. 2015, 400, 81–89. [Google Scholar] [CrossRef]
- Dai, C.; Tian, X.; Nie, Y.; Lin, H.-M.; Yang, C.; Han, B.; Wang, Y. Surface Facet of CuFeO2 Nanocatalyst: A Key Parameter for H2O2 Activation in Fenton-Like Reaction and Organic Pollutant Degradation. Environ. Sci. Technol. 2018, 52, 6518–6525. [Google Scholar] [CrossRef] [PubMed]
- Xiunan, C.; Ling, T.; Meifei, C.; Yijun, L.; Wei, W.; Junhao, L.; Yanjuan, Z.; Gan, T.; Huayu, H.; Zuqiang, H. Construction of a C-decorated and Cu-doped (Fe,Cu)S/CuFe2O4 solid solution for photo-Fenton degradation of hydrophobic organic contaminant: Enhanced electron transfer and adsorption capacity. Chemosphere 2022, 296, 134005. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, X.; Zhong, Y.; Zhu, J.; Zhu, S.; Yuan, P.; He, H.; Zhang, J. Heterogeneous UV/Fenton degradation of TBBPA catalyzed by titanomagnetite: Catalyst characterization, performance and degradation products. Water Res. 2012, 46, 4633–4644. [Google Scholar] [CrossRef]
- Chen, Z.; Bi, S.; Zhao, G.; Chen, Y.; Hu, Y. Enhanced degradation of triclosan by cobalt manganese spinel-type oxide activated peroxymonosulfate oxidation process via sulfate radicals and singlet oxygen: Mechanisms and intermediates identification. Sci. Total Environ. 2020, 711, 134715. [Google Scholar] [CrossRef]
- Wang, R.; An, H.; Zhang, H.; Zhang, X.; Feng, J.; Wei, T.; Ren, Y. High active radicals induced from peroxymonosulfate by mixed crystal types of CuFeO2 as catalysts in the water. Appl. Surf. Sci. 2019, 484, 1118–1127. [Google Scholar] [CrossRef]
- Hao, Y.-J.; Liu, B.; Tian, L.-G.; Li, F.-T.; Ren, J.; Liu, S.-J.; Liu, Y.; Zhao, J.; Wang, X.-J. Synthesis of {111} Facet-Exposed MgO with Surface Oxygen Vacancies for Reactive Oxygen Species Generation in the Dark. ACS Appl. Mater. Interfaces 2017, 9, 12687–12693. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Chen, X.; Hao, M.; Xiao, F.; Yang, S. Oxygen vacancy enhancing the Fe2O3-CeO2 catalysts in Fenton-like reaction for the sulfamerazine degradation under O2 atmosphere. Chemosphere 2019, 228, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.N.; Lago, R.M.; Fierro, J.L.G.; González, J. Hydrogen peroxide decomposition over Ln1−xAxMnO3 (Ln = La or Nd and A = K or Sr) perovskites. Appl. Catal. A Gen. 2001, 215, 245–256. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Mahdad, F.; Younesi, H.; Bahramifar, N.; Hadavifar, M. Optimization of Fenton and photo-Fenton-based advanced oxidation processes for post-treatment of composting leachate of municipal solid waste by an activated sludge process. KSCE J. Civ. Eng. 2016, 20, 2177–2188. [Google Scholar] [CrossRef]
- de Moura Gomes, L.; da Silva Duarte, J.L.; Pereira, N.M.; Martínez-Huitle, C.A.; Tonholo, J.; de Paiva e Silva Zanta, C.L. Development of a system for treatment of coconut industry wastewater using electrochemical processes followed by Fenton reaction. Water Sci. Technol. 2014, 69, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Al-Zurfi, S.K.L.; Alisaw, A.Y.; Al-Shafai, G.A.A. Anatomical and physiological effects of cadmium in aquatic plant Hydrilla Verticillata. Plant Arch. 2018, 18, 839–846. [Google Scholar]
- Reichwaldt, E.S.; Zheng, L.; Barrington, D.J.; Ghadouani, A. Acute toxicological response of Daphnia and Moina to hydrogen peroxide. J. Environ. Eng. 2012, 138, 607. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, P.M.; Andersen, C.V.B.; Schjelde, J.; Hansen, B.W. Tolerance of un-ionized ammonia in live feed cultures of the calanoid copepod Acartia tonsa Dana. Aquac. Res. 2015, 46, 420–431. [Google Scholar] [CrossRef]
- Arauzo, M.; Valladolid, M. Short-term harmful effects of unionised ammonia on natural populations of Moina micrura and Brachionus rubens in a deep waste treatment pond. Water Res. 2003, 37, 2547–2554. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Xu, H.; Li, Y.; Li, H.; Cheng, X.; Xia, J.; Xu, Y.; Cai, G. Visible-light-induced WO3/g-C3N4 composites with enhanced photocatalytic activity. Dalton Trans. 2013, 42, 8606–8616. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xu, Y.; Xie, M.; Xu, H.; He, M.; Xia, J.; Huang, L.; Li, H. Synthesis of magnetic CoFe2O4/g-C3N4 composite and its enhancement of photocatalytic ability under visible-light. Colloids Surf. A Physicochem. Eng. Asp. 2015, 478, 71–80. [Google Scholar] [CrossRef]
- Ghassemi, N.; Davarani, S.S.H.; Moazami, H.R. Cathodic electrosynthesis of CuFe2O4/CuO composite nanostructures for high performance supercapacitor applications. J. Mater. Sci. Mater. Electron. 2018, 29, 12573–12583. [Google Scholar] [CrossRef]
- Ghobadi, M.; Gharabaghi, M.; Abdollahi, H.; Boroumand, Z.; Moradian, M. MnFe2O4-graphene oxide magnetic nanoparticles as a high-performance adsorbent for rare earth elements: Synthesis, isotherms, kinetics, thermodynamics and desorption. J. Hazard. Mater. 2018, 351, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Goodgame, D.M.; Hussain, I.; White, A.J.; Williams, D.J. Synthesis and structure of a copper (II) melamine complex, [Cu(C3H6N6)(µ-OCH3)(ONO2)(HOCH3)]2, with direct Cu–melamine coordination. J. Chem. Soc. Dalton Trans. 1999, 2899–2900. [Google Scholar] [CrossRef]
- Wiles, A.B.; Bozzuto, D.; Cahill, C.L.; Pike, R.D. Copper (I) and (II) complexes of melamine. Polyhedron 2006, 25, 776–782. [Google Scholar] [CrossRef]
- Doddamani, J.S.; Hodlur, R.M.; Rabinal, M.K. Melamine assisted large-scale and rapid synthesis of porous copper oxide nanostructures. Emergent Mater. 2021. [Google Scholar] [CrossRef]
- Ansari, S.A.; Ansari, S.G.; Foaud, H.; Cho, M.H. Facile and sustainable synthesis of carbon-doped ZnO nanostructures towards the superior visible light photocatalytic performance. New J. Chem. 2017, 41, 9314–9320. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angkaew, A.; Sakulthaew, C.; Nimtim, M.; Imman, S.; Satapanajaru, T.; Suriyachai, N.; Kreetachat, T.; Comfort, S.; Chokejaroenrat, C. Enhanced Photo-Fenton Activity Using Magnetic Cu0.5Mn0.5Fe2O4 Nanoparticles as a Recoverable Catalyst for Degrading Organic Contaminants. Water 2022, 14, 3717. https://doi.org/10.3390/w14223717
Angkaew A, Sakulthaew C, Nimtim M, Imman S, Satapanajaru T, Suriyachai N, Kreetachat T, Comfort S, Chokejaroenrat C. Enhanced Photo-Fenton Activity Using Magnetic Cu0.5Mn0.5Fe2O4 Nanoparticles as a Recoverable Catalyst for Degrading Organic Contaminants. Water. 2022; 14(22):3717. https://doi.org/10.3390/w14223717
Chicago/Turabian StyleAngkaew, Athaphon, Chainarong Sakulthaew, Matura Nimtim, Saksit Imman, Tunlawit Satapanajaru, Nopparat Suriyachai, Torpong Kreetachat, Steve Comfort, and Chanat Chokejaroenrat. 2022. "Enhanced Photo-Fenton Activity Using Magnetic Cu0.5Mn0.5Fe2O4 Nanoparticles as a Recoverable Catalyst for Degrading Organic Contaminants" Water 14, no. 22: 3717. https://doi.org/10.3390/w14223717