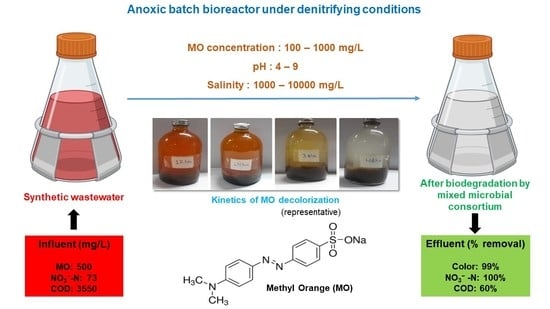
Effect of pH, Salinity, Dye, and Biomass Concentration on Decolourization of Azo Dye Methyl Orange in Denitrifying Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dye, Chemicals, and Nutrient Media
2.2. Seed Sludge
2.3. Enrichment of Biomass in SBR
2.4. Batch Studies
2.4.1. Effect of Dye Concentration
2.4.2. Effect of pH
2.4.3. Effect of Biomass Concentration
2.4.4. Effect of Salinity
2.4.5. Kinetic Studies
2.4.6. Evaluation of Biomass Growth
2.5. Analytical Techniques
3. Results and Discussion
3.1. Enrichment of Biomass in SBR
3.2. Effect of Dye Concentration
3.3. Effect of External Organic Carbon
3.4. Effect of Biomass Concentration
3.5. Effect of pH
3.6. Kinetics of MO Removal
3.7. Evaluation of Biomass Growth
3.8. Effect of Salinity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassaan, M.A.; El Nemr, A.; Hassaan, A. Health and environmental impacts of dyes: Mini review. Am. J. Environ. Sci. Eng. 2017, 1, 64–67. [Google Scholar] [CrossRef]
- Aggarwal, S. Indian dye yielding plants: Efforts and opportunities. In Natural Resources Forum; Wiley Online Library, 2021; pp. 63–86. [Google Scholar] [CrossRef]
- Shabbir, S.; Faheem, M.; Ali, N.; Kerr, P.G.; Wu, Y. Periphyton biofilms: A novel and natural biological system for the effective removal of sulphonated azo dye methyl orange by synergistic mechanism. Chemosphere 2017, 167, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Taheri, M.; Fallah, N.; Nasernejad, B. Which treatment procedure among electrocoagulation, biological, adsorption, and bio-adsorption processes performs best in azo dyes removal? Water Resour. Ind. 2022, 28, 100191. [Google Scholar] [CrossRef]
- Han, G.; Du, Y.; Huang, Y.; Wang, W.; Su, S.; Liu, B. Study on the removal of hazardous Congo red from aqueous solutions by chelation flocculation and precipitation flotation process. Chemosphere 2022, 289, 133109. [Google Scholar] [CrossRef]
- Arshadi, M.; Mehravar, M.; Amiri, M.; Faraji, A. Synthesis and adsorption characteristics of an heterogenized manganese nanoadsorbent towards methyl orange. J. Colloid Interface Sci. 2015, 440, 189–197. [Google Scholar] [CrossRef]
- Cockerham, C.; Caruthers, A.; McCloud, J.; Fortner, L.M.; Youn, S.; McBride, S.P. Azo-Dye-Functionalized Polycarbonate Membranes for Textile Dye and Nitrate Ion Removal. Micromachines 2022, 13, 577. [Google Scholar] [CrossRef]
- Marcus, R.N.; Joseph, C.G.; Taufiq-Yap, Y.H.; Soloi, S.; Hafiz, M.; Abd Majid, Z.J.; Ali, S.A.M.; Vijayan, V.; Pang, C.K. Current Trends on the Utilization of Ozonation Treatment Process for the Remediation of Dye Wastewater: A Short Review. Malays. J. Chem. 2022, 24, 113–124. [Google Scholar]
- Wakrim, A.; Zaroual, Z.; El Ghachtouli, S.; Jamal Eddine, J.; Azzi, M. Treatment and Degradation of Azo Dye Waste Industry by Electro-Fenton Process. Phys. Chem. Res. 2022, 10, 495–504. [Google Scholar] [CrossRef]
- Shao, D.; Li, W.; Wang, Z.; Yang, C.; Xu, H.; Yan, W.; Yang, L.; Wang, G.; Yang, J.; Feng, L. Variable activity and selectivity for electrochemical oxidation wastewater treatment using a magnetically assembled electrode based on Ti/PbO2 and carbon nanotubes. Sep. Purif. Technol. 2022, 301, 122008. [Google Scholar] [CrossRef]
- Selvaraj, V.; Swarna Karthika, T.; Mansiya, C.; Alagar, M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Parshetti, G.; Telke, A.; Kalyani, D.; Govindwar, S.P. Decolorization and detoxification of sulfonated azo dye methyl orange by Kocuria rosea MTCC 1532. J. Hazard. Mater. 2010, 176, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.K.Q.; Fung, K.Y.; Ng, K.M. Aerobic sludge granulation for simultaneous anaerobic decolorization and aerobic aromatic amines mineralization for azo dye wastewater treatment. Environ. Technol. 2018, 39, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.-H.; Gao, L.; Lee, H.-S.; Wang, A.-J. Mixed dye wastewater treatment in a bioelectrochemical system-centered process. Bioresour. Technol. 2020, 297, 122420. [Google Scholar] [CrossRef]
- Balapure, K.; Aghera, P.; Bhatt, N.; Madamwar, D. Community Synergism: Degradation of Triazine Dye Reactive Black 1 by Mixed Bacterial Cultures KND_PR under Microaerophilic and Aerobic Conditions. Environ. Process. 2019, 6, 713–739. [Google Scholar] [CrossRef]
- Aleem, M.; Cao, J.; Li, C.; Rashid, H.; Wu, Y.; Nawaz, M.I.; Abbas, M.; Akram, M.W. Coagulation-and adsorption-based environmental impact assessment and textile effluent treatment. Water Air Soil Pollut. 2020, 231, 45. [Google Scholar] [CrossRef]
- Swathi, D.; Sabumon, P.C.; Trivedi, A. Simultaneous decolorization and mineralization of high concentrations of methyl orange in an anoxic up-flow packed bed reactor in denitrifying conditions. J. Water Process Eng. 2021, 40, 101813. [Google Scholar] [CrossRef]
- Guo, G.; Hao, J.; Tian, F.; Liu, C.; Ding, K.; Xu, J.; Zhou, W.; Guan, Z. Decolorization and detoxification of azo dye by halo-alkaliphilic bacterial consortium: Systematic investigations of performance, pathway and metagenome. Ecotoxicol. Environ. Saf. 2020, 204, 111073. [Google Scholar] [CrossRef]
- Hou, C.; Shen, J.; Jiang, X.; Zhang, D.; Sun, X.; Li, J.; Han, W.; Liu, X.; Wang, L. Enhanced anoxic biodegradation of pyridine coupled to nitrification in an inner loop anoxic/oxic-dynamic membrane bioreactor (A/O-DMBR). Bioresour. Technol. 2018, 267, 626–633. [Google Scholar] [CrossRef]
- Saini, R.D. Textile organic dyes: Polluting effects and elimination methods from textile waste water. Int. J. Chem. Eng. Res. 2017, 9, 121–136. [Google Scholar]
- Manogaran, M.; Yasid, N.A.; Othman, A.R.; Gunasekaran, B.; Halmi, M.I.E.; Shukor, M.Y.A. Biodecolourisation of Reactive Red 120 as a Sole Carbon Source by a Bacterial Consortium—Toxicity Assessment and Statistical Optimisation. Int. J. Environ. Res. Public Health 2021, 18, 2424. [Google Scholar] [CrossRef] [PubMed]
- Metcalf; Eddy; Abu-Orf, M.; Bowden, G.; Burton, F.L.; Pfrang, W.; Stensel, H.D.; Tchobanoglous, G.; Tsuchihashi, R.; AECOM. Wastewater Engineering: Treatment and Resource Recovery; McGraw Hill Education: New York, NY, USA, 2014. [Google Scholar]
- APHA; AWWA; WPCF. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Desireddy, S.; Raghupatruni Lakshmi, M.; Pothanamkandathil Chacko, S.; Mehta, A. Development of an anoxic nitrification-denitrification process in a granulated nanoscale oxyhydroxides of Fe packed bed reactor for the simultaneous removal of NH4+-N and COD. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100412. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, P.; Iyengar, L. Bacterial decolorization and degradation of azo dyes. Int. Biodeterior. Biodegrad. 2007, 59, 73–84. [Google Scholar] [CrossRef]
- Al-Amrani, W.A.; Lim, P.-E.; Seng, C.-E.; Wan Ngah, W.S. Factors affecting bio-decolorization of azo dyes and COD removal in anoxic–aerobic REACT operated sequencing batch reactor. J. Taiwan Inst. Chem. Eng. 2014, 45, 609–616. [Google Scholar] [CrossRef]
- Patel, D.K.; Tipre, D.R.; Dave, S.R. Enzyme mediated bacterial biotransformation and reduction in toxicity of 1:2 chromium complex AB193 and AB194 dyes. J. Taiwan Inst. Chem. Eng. 2017, 77, 1–9. [Google Scholar] [CrossRef]
- Nikam, M.; Patil, S.; Patil, U.; Khandare, R.; Govindwar, S.; Chaudhari, A. Biodegradation and detoxification of azo solvent dye by ethylene glycol tolerant ligninolytic ascomycete strain of Pseudocochliobolus verruculosus NFCCI 3818. Biocatal. Agric. Biotechnol. 2017, 9, 209–217. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, P.-F.; Du, L.-N.; Wang, G.; Jia, X.-M.; Zhao, Y.-H. Biodegradation of methyl red by Bacillus sp. strain UN2: Decolorization capacity, metabolites characterization, and enzyme analysis. Environ. Sci. Pollut. Res. 2014, 21, 6136–6145. [Google Scholar] [CrossRef]
- Nouren, S.; Bhatti, H.N.; Iqbal, M.; Bibi, I.; Nazar, N.; Iqbal, D.N.; Kanwal, Q.; Kausar, A.; Hussain, F. Redox Mediators Assisted-degradation of Direct Yellow 4. Pol. J. Environ. Stud. 2017, 26, 2885–2890. [Google Scholar] [CrossRef]
- Chang, J.-S.; Chou, C.; Lin, Y.-C.; Lin, P.-J.; Ho, J.-Y.; Lee Hu, T. Kinetic characteristics of bacterial azo-dye decolorization by Pseudomonas luteola. Water Res. 2001, 35, 2841–2850. [Google Scholar] [CrossRef]
- Maniyam, M.N.; Ibrahim, A.L.; Cass, A.E. Decolourization and biodegradation of azo dye methyl red by Rhodococcus strain UCC 0016. Environ. Technol. 2020, 41, 71–85. [Google Scholar] [CrossRef]
- Ayed, L.; Mahdhi, A.; Cheref, A.; Bakhrouf, A. Decolorization and degradation of azo dye Methyl Red by an isolated Sphingomonas paucimobilis: Biotoxicity and metabolites characterization. Desalination 2011, 274, 272–277. [Google Scholar] [CrossRef]
- Chen, B.-Y. Understanding decolorization characteristics of reactive azo dyes by Pseudomonas luteola: Toxicity and kinetics. Process Biochem. 2002, 38, 437–446. [Google Scholar] [CrossRef]
- Ong, S.-A.; Toorisaka, E.; Hirata, M.; Hano, T. Decolorization of Orange II using an anaerobic sequencing batch reactor with and without co-substrates. J. Environ. Sci. 2012, 24, 291–296. [Google Scholar] [CrossRef]
- Işık, M.; Sponza, D.T. Effects of alkalinity and co-substrate on the performance of an upflow anaerobic sludge blanket (UASB) reactor through decolorization of Congo Red azo dye. Bioresour. Technol. 2005, 96, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, J.Q.; Zheng, X.W.; Tian, Y.; Xiong, X.J.; Zheng, T.L. Bacterial decolorization and degradation of the reactive dye Reactive Red 180 by Citrobacter sp. CK3. Int. Biodeterior. Biodegrad. 2009, 63, 395–399. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Danish, M.; Singh, R.S.; Rafatullah, M.; HPS, A.K. Exploiting microbial biomass in treating azo dyes contaminated wastewater: Mechanism of degradation and factors affecting microbial efficiency. J. Water Process Eng. 2021, 43, 102255. [Google Scholar] [CrossRef]
- Pourbabaee, A.; Malekzadeh, F.; Sarbolouki, M.; Najafi, F. Aerobic decolorization and detoxification of a disperse dye in textile effluent by a new isolate of Bacillus sp. Biotechnol. Bioeng. 2006, 93, 631–635. [Google Scholar] [CrossRef]
- Garg, S.K.; Tripathi, M.; Singh, S.K.; Tiwari, J.K. Biodecolorization of textile dye effluent by Pseudomonas putida SKG-1 (MTCC 10510) under the conditions optimized for monoazo dye orange II color removal in simulated minimal salt medium. Int. Biodeterior. Biodegrad. 2012, 74, 24–35. [Google Scholar] [CrossRef]
- Garg, S.K.; Tripathi, M. Process parameters for decolorization and biodegradation of orange II (Acid Orange 7) in dye-simulated minimal salt medium and subsequent textile effluent treatment by Bacillus cereus (MTCC 9777) RMLAU1. Environ. Monit. Assess. 2013, 185, 8909–8923. [Google Scholar] [CrossRef]
- Pearce, C.I.; Lloyd, J.R.; Guthrie, J.T. The removal of colour from textile wastewater using whole bacterial cells: A review. Dye. Pigment. 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Dawkar, V.V.; Jadhav, U.U.; Ghodake, G.S.; Govindwar, S.P. Effect of inducers on the decolorization and biodegradation of textile azo dye Navy blue 2GL by Bacillus sp. VUS. Biodegradation 2009, 20, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Masarbo, R.S.; Karegoudar, T. Decolourisation of toxic azo dye Fast Red E by three bacterial strains: Process optimisation and toxicity assessment. Int. J. Environ. Anal. Chem. 2020, 102, 2686–2696. [Google Scholar] [CrossRef]
- Wu, H.; Sun, Q.; Sun, Y.; Zhou, Y.; Wang, J.; Hou, C.; Jiang, X.; Liu, X.; Shen, J. Co-metabolic enhancement of 1H-1,2,4-triazole biodegradation through nitrification. Bioresour. Technol. 2019, 271, 236–243. [Google Scholar] [CrossRef]
- Anjali, G.; Sabumon, P.C. Unprecedented development of anammox in presence of organic carbon using seed biomass from a tannery Common Effluent Treatment Plant (CETP). Bioresour. Technol. 2014, 153, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, J.; Lu, H.; Jin, R.; Zhou, J.; Zhang, L. Effects of reduction products of ortho-hydroxyl substituted azo dyes on biodecolorization of azo dyes. J. Hazard. Mater. 2009, 171, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.; Lee, K.; Chang, Y. Biodegradation of mono azo dye-Reactive Orange 16 by acclimatizing biomass systems under an integrated anoxic-aerobic REACT sequencing batch moving bed biofilm reactor. J. Water Process Eng. 2020, 36, 101268. [Google Scholar] [CrossRef]
- El Bouraie, M.; El Din, W.S. Biodegradation of Reactive Black 5 by Aeromonas hydrophila strain isolated from dye-contaminated textile wastewater. Sustain. Environ. Res. 2016, 26, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, M.; Gallert, C.; Winter, J. Treatment of phenolic wastewater in an anaerobic fixed bed reactor (AFBR)—Recovery after shock loading. J. Hazard. Mater. 2009, 162, 1330–1339. [Google Scholar] [CrossRef]
- Gao, W.; Leung, K.; Qin, W.; Liao, B. Effects of temperature and temperature shock on the performance and microbial community structure of a submerged anaerobic membrane bioreactor. Bioresour. Technol. 2011, 102, 8733–8740. [Google Scholar] [CrossRef]
- Mirbolooki, H.; Amirnezhad, R.; Pendashteh, A.R. Treatment of high saline textile wastewater by activated sludge microorganisms. J. Appl. Res. Technol. 2017, 15, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Liu, G.; Zhou, J.; Fu, Q.S. Effects of redox mediators on azo dye decolorization by Shewanella algae under saline conditions. Bioresour. Technol. 2014, 151, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Li, X.; Tian, F.; Liu, T.; Yang, F.; Ding, K.; Liu, C.; Chen, J.; Wang, C. Azo dye decolorization by a halotolerant consortium under microaerophilic conditions. Chemosphere 2020, 244, 125510. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, G.; Zhou, J.; Shiang Fu, Q.; Wang, G. Azo dye decolorization by Shewanella aquimarina under saline conditions. Bioresour. Technol. 2012, 114, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, W.; Wang, Y.; Wu, L.; Liu, X.; Yan, T.; Alm, E.; Arkin, A.; Thompson, D.K.; Fields, M.W.; et al. Transcriptome analysis of Shewanella oneidensis MR-1 in response to elevated salt conditions. J. Bacteriol. 2005, 187, 2501–2507. [Google Scholar] [CrossRef] [PubMed]











| Phase | Day of Operation | No. of Cycles | MO (mg/L) | COD (mg/L) | NO3-N * (mg/L) | Cycle Time (d) |
|---|---|---|---|---|---|---|
| I | 1–90 | 1–30 | 500 | 3550 ± 280 | 73 ± 2 | 3 |
| II | 91–120 | 31–45 | 500 | 3550 ± 280 | 73 ± 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivedi, A.; Desireddy, S.; Chacko, S.P. Effect of pH, Salinity, Dye, and Biomass Concentration on Decolourization of Azo Dye Methyl Orange in Denitrifying Conditions. Water 2022, 14, 3747. https://doi.org/10.3390/w14223747
Trivedi A, Desireddy S, Chacko SP. Effect of pH, Salinity, Dye, and Biomass Concentration on Decolourization of Azo Dye Methyl Orange in Denitrifying Conditions. Water. 2022; 14(22):3747. https://doi.org/10.3390/w14223747
Chicago/Turabian StyleTrivedi, Aditi, Swathi Desireddy, and Sabumon Pothanamkandathil Chacko. 2022. "Effect of pH, Salinity, Dye, and Biomass Concentration on Decolourization of Azo Dye Methyl Orange in Denitrifying Conditions" Water 14, no. 22: 3747. https://doi.org/10.3390/w14223747