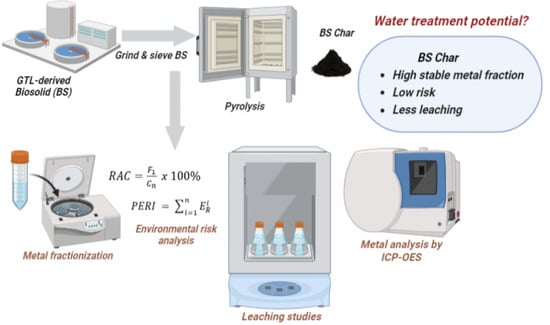
Potential of GTL-Derived Biosolids for Water Treatment: Fractionization, Leachate, and Environmental Risk Analysis
Abstract
:1. Introduction
- Characterization of the biosolids (BS) and pyrolyzed BS: BS char (BSC) samples.
- Determination of the metal content in the samples using the acid digestion method.
- Fractionization of some common metals present in both samples following the Community Bureau of Reference (BCR) sequential extraction procedure.
- Leaching of metals in water for both samples following the TCLP (Toxicity Characteristic Leaching Procedure).
- Environmental risk assessment based on the Risk Assessment Code (RAC) and Potential Ecological Risk Index (PERI) methods.
2. Materials and Methods
2.1. Pyrolysis
2.2. Feedstock and Biochar Characterization
2.2.1. Proximate and Ultimate Analysis
2.2.2. pH
2.2.3. Calorific Value
2.2.4. XRD
2.2.5. Surface Area and Pore Volume
2.2.6. Surface Zeta Potential
2.2.7. SEM-EDS
2.2.8. Metal Analysis by Acid Digestion
2.3. Metal Fractionization Following the BCR Procedure
2.4. Environmental Risk Analysis
2.5. Leaching Analysis Following TCLP
3. Results and Discussion
3.1. Characterization of Samples
3.2. Fractionization of BS Samples
3.3. Risk Analysis Studies
3.4. Leaching Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| American Society for Testing and Materials | ASTM |
| Community Bureau of Reference | BCR |
| Brunauer–Emmett–Teller | BET |
| Biosolid | BS |
| Biosolid biochar | BSC |
| Gas-to-Liquids | GTL |
| Scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) | SEM-EDS |
| Toxicity Characteristic Leaching Procedure | TCLP |
| X-ray diffractometer | XRD |
References
- Mariyam, S.; Shahbaz, M.; Al-Ansari, T.; Mackey, H.R.; McKay, G. A Critical Review on Co-Gasification and Co-Pyrolysis for Gas Production. Renew. Sustain. Energy Rev. 2022, 161, 112349. [Google Scholar] [CrossRef]
- Zuhara, S.; Mackey, H.R.; Al-Ansari, T.; McKay, G. A Review of Prospects and Current Scenarios of Biomass Co-Pyrolysis for Water Treatment. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Ghodke, P.K.; Sharma, A.K.; Pandey, J.K.; Chen, W.H.; Patel, A.; Ashokkumar, V. Pyrolysis of Sewage Sludge for Sustainable Biofuels and Value-Added Biochar Production. J. Environ. Manag. 2021, 298, 113450. [Google Scholar] [CrossRef] [PubMed]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels: A Review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef] [PubMed]
- Batista Meneses, D.; Montes de Oca-Vásquez, G.; Vega-Baudrit, J.R.; Rojas-Álvarez, M.; Corrales-Castillo, J.; Murillo-Araya, L.C. Pretreatment Methods of Lignocellulosic Wastes into Value-Added Products: Recent Advances and Possibilities. Biomass Convers. Biorefin. 2022, 12, 547–564. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, M.P.S.; Makkar, H.P.S. Wastes to Worth: Value Added Products from Fruit and Vegetable Wastes. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2015, 10. [Google Scholar] [CrossRef]
- Subramanian, K.; Sarkar, M.K.; Wang, H.; Qin, Z.H.; Chopra, S.S.; Jin, M.; Kumar, V.; Chen, C.; Tsang, C.W.; Lin, C.S.K. An Overview of Cotton and Polyester, and Their Blended Waste Textile Valorisation to Value-Added Products: A Circular Economy Approach–Research Trends, Opportunities and Challenges. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3921–3942. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Mackey, H.R.; Al-Ansari, T.; McKay, G. Pyrolysis of Biosolids to Produce Biochars: A Review. Sustainability 2022, 14, 9626. [Google Scholar] [CrossRef]
- Hurynovich, A.; Kwietniewski, M.; Romanovski, V. Evaluation of the Possibility of Utilization of Sewage Sludge from a Wastewater Treatment Plant—Case Study. Desalin. Water Treat. 2021, 227, 16–25. [Google Scholar] [CrossRef]
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable Management of Water Treatment Sludge through 3′R’ Concept. J. Clean. Prod. 2016, 124, 1–13. [Google Scholar] [CrossRef]
- Lin, L.; Xu, X.; Papelis, C.; Cath, T.Y.; Xu, P. Sorption of Metals and Metalloids from Reverse Osmosis Concentrate on Drinking Water Treatment Solids. Sep. Purif. Technol. 2014, 134, 37–45. [Google Scholar] [CrossRef]
- Kogbara, R.B.; Yiming, W.; Iyengar, S.R.; Onwusogh, U.C.; Youssef, K.; Al-Ansary, M.; Sunifar, P.A.; Arora, D.; Al-Sharshani, A.; Abdalla, O.A.E.; et al. Recycling Industrial Biosludge for Buffel Grass Production in Qatar: Impact on Soil, Leachate and Plant Characteristics. Chemosphere 2020, 247, 125886. [Google Scholar] [CrossRef]
- Hosseini Koupaie, E.; Eskicioglu, C. Health Risk Assessment of Heavy Metals through the Consumption of Food Crops Fertilized by Biosolids: A Probabilistic-Based Analysis. J. Hazard. Mater. 2015, 300, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Flury, M. Effects of Freezing–Thawing and Wetting–Drying on Heavy Metal Leaching from Biosolids. Water Environ. Res. 2019, 91, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.; Nielsen, C. Leaching from Land Disposed Municipal Composts: 1. Organic Matter. Waste Manag. Res. 1983, 1, 83–94. [Google Scholar] [CrossRef]
- Yang, Y.; He, Z.; Stoffella, P.J.; Banks, D.J.; Yang, Y.; He, Z.; Graetz, D.A.; Yang, X. Leaching Behavior of Heavy Metals In Biosolids Amended Sandy Soils. Compost Sci. Util. 2008, 16, 144–151. [Google Scholar] [CrossRef]
- Brown, S.; Beecher, N.; Carpenter, A. Calculator Tool for Determining Greenhouse Gas Emissions for Biosolids Processing and End Use. Environ. Sci. Technol. 2010, 44, 9509–9515. [Google Scholar] [CrossRef]
- Kanteraki, A.E.; Isari, E.A.; Svarnas, P.; Kalavrouziotis, I.K. Biosolids: The Trojan Horse or the Beautiful Helen for Soil Fertilization? Sci. Total Environ. 2022, 839, 156270. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Wade, P.; Bolan, N. Assessment of the Fertilizer Potential of Biochars Produced from Slow Pyrolysis of Biosolid and Animal Manures. J. Anal. Appl. Pyrolysis 2021, 155, 105043. [Google Scholar] [CrossRef]
- He, Y.D.; Zhai, Y.B.; Li, C.T.; Yang, F.; Chen, L.; Fan, X.P.; Peng, W.F.; Fu, Z.M. The Fate of Cu, Zn, Pb and Cd during the Pyrolysis of Sewage Sludge at Different Temperatures. Environ. Technol. 2010, 31, 567–574. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Gui, C.; Li, P.; Zhang, J.; Zhong, H.; Wei, Y. Chemical Forms and Risk Assessment of Heavy Metals in Sludge-Biochar Produced by Microwave-Induced Low Temperature Pyrolysis. RSC Adv. 2016, 6, 101960–101967. [Google Scholar] [CrossRef]
- Tang, J.; Tang, H.; Sima, W.; Wang, H.; Zou, D.; Qiu, B.; Qu, J.; Liang, R.; Dong, J.; Liao, Y.; et al. Heavy Metal Pollution Level and Potential Ecological Risk Assessment of Sludge Landfill. Environ. Prog. Sustain. Energy 2022, 41, 1–10. [Google Scholar] [CrossRef]
- Ukwatta, A.; Mohajerani, A. Leachate Analysis of Green and Fired-Clay Bricks Incorporated with Biosolids. Waste Manag. 2017, 66, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Nuagah, M.B.; Boakye, P.; Oduro-Kwarteng, S.; Sokama-Neuyam, Y.A. Valorization of Faecal and Sewage Sludge via Pyrolysis for Application as Crop Organic Fertilizer. J. Anal. Appl. Pyrolysis 2020, 151, 104903. [Google Scholar] [CrossRef]
- Bolognesi, S.; Bernardi, G.; Callegari, A.; Dondi, D.; Capodaglio, A.G. Biochar Production from Sewage Sludge and Microalgae Mixtures: Properties, Sustainability and Possible Role in Circular Economy. Biomass Convers. Biorefin. 2021, 11, 289–299. [Google Scholar] [CrossRef]
- Phoungthong, K.; Zhang, H.; Shao, L.M.; He, P.J. Leaching Characteristics and Phytotoxic Effects of Sewage Sludge Biochar. J. Mater. Cycles Waste Manag. 2018, 20, 2089–2099. [Google Scholar] [CrossRef]
- Gusiatin, Z.M.; Kulikowska, D. The Usability of the IR, RAC and MRI Indices of Heavy Metal Distribution to Assess the Environmental Quality of Sewage Sludge Composts. Waste Manag. 2014, 34, 1227–1236. [Google Scholar] [CrossRef]
- Nkinahamira, F.; Suanon, F.; Chi, Q.; Li, Y.; Feng, M.; Huang, X.; Yu, C.P.; Sun, Q. Occurrence, Geochemical Fractionation, and Environmental Risk Assessment of Major and Trace Elements in Sewage Sludge. J. Environ. Manage. 2019, 249, 109427. [Google Scholar] [CrossRef]
- Latosińska, J.; Kowalik, R.; Gawdzik, J. Risk Assessment of Soil Contamination with Heavy Metals from Municipal Sewage Sludge. Appl. Sci. 2021, 11, 548. [Google Scholar] [CrossRef]
- Kowalik, R.; Latosińska, J.; Gawdzik, J. Risk Analysis of Heavy Metal Accumulation from Sewage Sludge of Selected Wastewater Treatment Plants in Poland. Water 2021, 13, 2070. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Li, Y.; Han, L. Influence of Pyrolysis Temperature on Chemical Speciation, Leaching Ability, and Environmental Risk of Heavy Metals in Biochar Derived from Cow Manure. Bioresour. Technol. 2020, 302, 122850. [Google Scholar] [CrossRef]
- Wang, A.; Zou, D.; Zeng, X.; Chen, B.; Zheng, X.; Li, L.; Zhang, L.; Xiao, Z.; Wang, H. Speciation and Environmental Risk of Heavy Metals in Biochars Produced by Pyrolysis of Chicken Manure and Water-Washed Swine Manure. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, B.; Liu, H.; Zhao, Y.; Li, L. Effects of Pyrolysis Temperature on Biochar’s Characteristics and Speciation and Environmental Risks of Heavy Metals in Sewage Sludge Biochars. Environ. Technol. Innov. 2022, 26, 102288. [Google Scholar] [CrossRef]
- Chanaka, C.U.; Veksha, A.; Giannis, A.; Liang, Y.N.; Lisak, G.; Hu, X.; Lim, T.T. Insights into the Speciation of Heavy Metals during Pyrolysis of Industrial Sludge. Sci. Total Environ. 2019, 691, 232–242. [Google Scholar] [CrossRef]







| Risk Analysis | Range | Level |
|---|---|---|
| Risk Assessment Code (RAC) [21] | Less than 1% | No risk (NR) |
| 1–10% | Low risk | |
| 11–30% | Medium risk | |
| 31–50% | High risk | |
| >50% | Very high risk | |
| The potential risk of individual heavy metal, [22] | <40 | Low risk |
| 40 ≤ Er < 80 | Moderate risk | |
| 80 ≤ Er < 160 | Considerable | |
| 160 ≤ Er < 320 | High potential | |
| Er ≥ 320 | Very high | |
| Sum of the potential risk of individual heavy metal, PERI [22] | <150 | Low risk |
| 150 ≤ RI < 300 | Moderate risk | |
| 300 ≤ RI < 600 | Considerable | |
| RI > 600 | Very high |
| Sample | pH | Surface Area (m2/g) | Pore Volume (cm3/g) | Calorific Value (MJ/kg) | Surface Charge (mV) | Yield (%) |
|---|---|---|---|---|---|---|
| BS | 8.42 | 0.0100 | 0.0210 | 19.4 | −29.5 | N/A |
| BSC | 9.23 | 0.0185 | 0.0137 | 21.4 | −25.3 | ≈45 |
| Proximate analysis (% w/w) | ||||||
| Sample | Moisture | Volatile matter | Ash | Fixed carbon | ||
| BS | 12.29 | 47.47 | 26.07 | 14.16 | ||
| BSC | 4.82 | 15.75 | 37.57 | 41.86 | ||
| Ultimate analysis (% w/w) | ||||||
| Sample | C | H | O | N | S | |
| BS | 33.69 | 6.18 | 29.03 | 5.03 | - | |
| BSC | 43.87 | 2.90 | 4.07 | 5.16 | 6.43 | |
| Sample | BS | BSC | ||
|---|---|---|---|---|
| Element | Mass (%) | Atom (%) | Mass (%) | Atom (%) |
| Carbon | 40.3 | 53.0 | 58.2 | 68.2 |
| Nitrogen | 5.60 | 6.31 | 8.64 | 8.68 |
| Oxygen | 33.0 | 32.5 | 21.4 | 18.8 |
| Sodium | 0.280 | 0.200 | 0.920 | 0.570 |
| Magnesium | 0.140 | 0.0900 | 0.170 | 0.100 |
| Aluminum | 0.160 | 0.0900 | 0.190 | 0.100 |
| Silicon | 1.66 | 0.850 | 0.0500 | 0.0200 |
| Phosphorous | 1.58 | 0.780 | 1.49 | 0.680 |
| Sulfur | 0.120 | 0.0500 | 1.45 | 0.640 |
| Chlorine | 0.230 | 0.0900 | 0.110 | 0.0500 |
| Potassium | 10.1 | 3.98 | 0.470 | 0.170 |
| Calcium | 0.270 | 0.0800 | 2.56 | 0.900 |
| Iron | 6.53 | 1.85 | 4.29 | 1.08 |
| Sample | Metal Concentration (mg/g) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Ba | Co | Cr | Cu | K | Mn | Ni | Pb | Sr | Ca | Zn | Fe | |
| BS | 0.410 | 0.0100 | 0.0500 | 0.0300 | 0.0200 | 0.160 | 0.740 | 0.0300 | 0.0100 | 0.0300 | 0.540 | 0.580 | 46.2 |
| BSC | 0.460 | 0.0100 | 0.0600 | 0.0500 | 0.0400 | 0.200 | 0.930 | 0.0400 | 0.0200 | 0.0400 | 0.610 | 0.770 | 52.5 |
| Sample | Acid Digested (mg/g) | BCR (mg/g) | Standard Deviation |
|---|---|---|---|
| BS | 48.8 | 43.7 | 3.58 |
| BSC | 55.7 | 47.4 | 5.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuhara, S.; Pradhan, S.; Pasha, M.; McKay, G. Potential of GTL-Derived Biosolids for Water Treatment: Fractionization, Leachate, and Environmental Risk Analysis. Water 2022, 14, 4016. https://doi.org/10.3390/w14244016
Zuhara S, Pradhan S, Pasha M, McKay G. Potential of GTL-Derived Biosolids for Water Treatment: Fractionization, Leachate, and Environmental Risk Analysis. Water. 2022; 14(24):4016. https://doi.org/10.3390/w14244016
Chicago/Turabian StyleZuhara, Shifa, Snigdhendubala Pradhan, Mujaheed Pasha, and Gordon McKay. 2022. "Potential of GTL-Derived Biosolids for Water Treatment: Fractionization, Leachate, and Environmental Risk Analysis" Water 14, no. 24: 4016. https://doi.org/10.3390/w14244016