Fine-Scale Assessment of Greenhouse Gases Fluxes from a Boreal Peatland Pond
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Gas Flux Measurement
2.3. Methane Ebullition
2.4. Environmental Parameters and Plant Biomass
2.5. Collection and Analysis of Water and Sediment/Soil Samples
2.6. Statistical Analysis
3. Results
3.1. Chemical Properties of Sediment/Soil
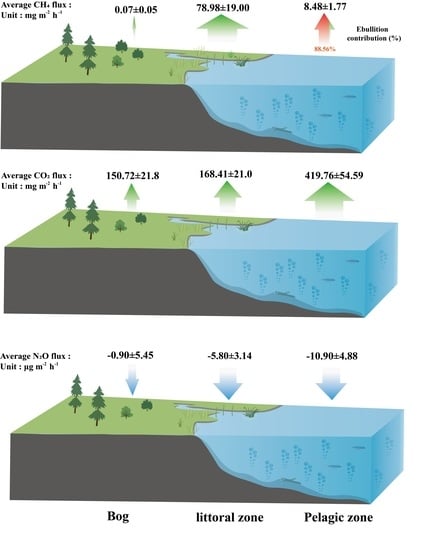
3.2. CH4 Fluxes
3.3. CO2 Fluxes
3.4. N2O Fluxes
4. Discussion
4.1. CH4 Fluxes
4.1.1. CH4 Fluxes within the Pond
4.1.2. The Littoral Zone Was a Hotspot for CH4 Fluxes Compared to Pond and Adjacent Bog
4.2. CO2 Fluxes
4.3. N2O Fluxes
4.4. Implications for the Peatland Pond System from Peatland Landscapes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC, Intergovernmental Panel on Climate Change. The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V.P., Zhai, A., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. (in press) [Google Scholar]
- World Meteorological Organization (WMO). WMO Greenhouse Gas Bulletin (GHG Bulletin)—No. 15: The State of Greenhouse Gases in the Atmosphere Based on Global Observations through 2018; WHO: Geneva, Switzerland, 2019; Available online: https://library.wmo.int/index.php?lvl=notice_display&id=21620 (accessed on 1 January 2021).
- Verpoorter, C.; Kutser, T.; Seekell, D.A.; Tranvik, L.J. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 2014, 41, 6396–6402. [Google Scholar] [CrossRef]
- Raymond, P.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef] [Green Version]
- DelSontro, T.; Beaulieu, J.; Downing, J. Greenhouse gas emissions from lakes and impoundments: Upscaling in the face of global change. Limnol. Oceanogr. Lett. 2018, 3, 64–75. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Borges, A.V.; Deemer, B.R.; Holgerson, M.A.; Liu, S.; Song, C.; Melack, J.; Raymond, P.A.; Duarte, C.M.; Allen, G.H.; et al. Half of global methane emissions come from highly variable aquatic ecosystem sources. Nat. Geosci. 2021, 14, 225–230. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 172–185. [Google Scholar] [CrossRef] [Green Version]
- Holgerson, M.A.; Raymond, P.A. Large contribution to inland water CO2 and CH4 emissions from very small ponds. Nat. Geosci. 2016, 9, 222–226. [Google Scholar] [CrossRef]
- Martinsen, K.T.; Kragh, T.; Sand-Jensen, K. Carbon dioxide efflux and ecosystem metabolism of small forest lakes. Aquat. Sci. 2019, 82, 9. [Google Scholar] [CrossRef]
- Woolway, R.I.; Kraemer, B.M.; Lenters, J.D.; Merchant, C.J.; O’Reilly, C.M.; Sharma, S. Global lake responses to climate change. Nat. Rev. Earth Environ. 2020, 1, 388–403. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef] [Green Version]
- Huttunen, J.T.; Alm, J.; Liikanen, A.; Juutinen, S.; Larmola, T.; Hammar, T.; Silvola, J.; Martikainen, P.J. Fluxes of methane, carbon dioxide and nitrous oxide in boreal lakes and potential anthropogenic effects on the aquatic greenhouse gas emissions. Chemosphere 2003, 52, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Bastviken, D.; Cole, J.J.; Pace, M.L.; Van de Bogert, M.C. Fates of methane from different lake habitats: Connecting whole-lake budgets and CH4 emissions. J. Geophys. Res. Biogeosci. 2008, 113(G2), 61–74. [Google Scholar] [CrossRef]
- Kortelainen, P.; Larmola, T.; Rantakari, M.; Juutinen, S.; Alm, J.; Martikainen, P.J. Lakes as nitrous oxide sources in the boreal landscape. Glob. Change Biol. 2020, 26, 1432–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soued, C.; del Giorgio, P.A.; Maranger, R. Nitrous oxide sinks and emissions in boreal aquatic networks in Québec. Nat. Geosci. 2016, 9, 116–120. [Google Scholar] [CrossRef]
- Cole, J.; Caraco, N. Atmospheric exchange of carbon dioxide in a low-wind oligotrophic lake measured by the addition of SF6. Limnol. Oceanogr. 1998, 43, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Liu, X.; Yi, N.; Wang, Y.; Guo, J.; Zhang, Z.; Yan, S. Estimation of N2 and N2O ebullition from eutrophic water using an improved bubble trap device. Ecol. Eng. 2013, 57, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Sepulveda-Jauregui, A.; Walter Anthony, K.; Martinez-Cruz, K.; Greene, S.; Thalasso, F. Methane and carbon dioxide emissions from 40 lakes along a north–south latitudinal transect in Alaska. Biogeosciences 2015, 12, 3197–3223. [Google Scholar] [CrossRef] [Green Version]
- Bastviken, D.; Cole, J.; Pace, M.; Tranvik, L. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Glob. Biogeochem. Cycles 2004, 18. [Google Scholar] [CrossRef]
- Li, M.; Peng, C.; Zhu, Q.; Zhou, X.; Yang, G.; Song, X.; Zhang, K. The significant contribution of lake depth in regulating global lake diffusive methane emissions. Water Res. 2020, 172, 115465. [Google Scholar] [CrossRef]
- Baulch, H.M.; Dillon, P.J.; Maranger, R.; Schiff, S.L. Diffusive and ebullitive transport of methane and nitrous oxide from streams: Are bubble-mediated fluxes important? J. Geophys. Res. Biogeosci. 2011, 116. [Google Scholar] [CrossRef]
- Saunois, M.; Bousquet, P.; Poulter, B.; Peregon, A.; Ciais, P.; Canadell, J.; Dlugokencky, E.; Etiope, G.; Bastviken, D.; Houweling, S.; et al. The global methane budget 2000–2012. Earth Syst. Sci. Data 2016, 8, 697–751. [Google Scholar] [CrossRef]
- Desrosiers, K.; DelSontro, T.; del Giorgio, P.A. Disproportionate contribution of vegetated habitats to the CH4 and CO2 budgets of a boreal lake. Ecosystems 2022, 25, 1522–1541. [Google Scholar] [CrossRef]
- Woodman, S.G.; Khoury, S.; Fournier, R.E.; Emilson, E.J.S.; Gunn, J.M.; Rusak, J.A.; Tanentzap, A.J. Forest defoliator outbreaks alter nutrient cycling in northern waters. Nat. Commun. 2021, 12, 6355. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Burd, K.; Kuhn, M.; Estop-Aragonés, C.; Tank, S.E.; Olefeldt, D. Aged soils contribute little to contemporary carbon cycling downstream of thawing permafrost peatlands. Glob. Change Biol. 2021, 27, 5368–5382. [Google Scholar] [CrossRef]
- Groffman, P.; Gold, A.; Addy, K. Nitrous oxide production in riparian zones and its importance to national emission inventories. Chemosphere—Glob. Change Sci. 2000, 2, 291–299. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Yin, C.; Wang, Y.; Lu, J. Littoral zones as the “hotspots” of nitrous oxide (N2O) emission in a hyper-eutrophic lake in China. Atmos. Environ. 2006, 40, 5522–5527. [Google Scholar] [CrossRef]
- Vesterinen, J.; Devlin, S.P.; Syväranta, J.; Jones, R.I. Influence of littoral periphyton on whole-lake metabolism relates to littoral vegetation in humic lakes. Ecology 2017, 98, 3074–3085. [Google Scholar] [CrossRef] [Green Version]
- Juutinen, S.; Alm, J.; Larmola, T.; Huttunen, J.T.; Morero, M.; Martikainen, P.J.; Silvola, J. Major implication of the littoral zone for methane release from boreal lakes. Glob. Biogeochem. Cycles 2003, 17. [Google Scholar] [CrossRef]
- Kankaala, P.; Huotari, J.; Tulonen, T.; Ojala, A. Lake-size dependent physical forcing drives carbon dioxide and methane effluxes from lakes in a boreal landscape. Limnol. Oceanogr. 2013, 58, 1915–1930. [Google Scholar] [CrossRef]
- Sun, X.; Wang, H.; Song, C.; Jin, X.; Richardson, C.; Cai, T. Response of methane and nitrous oxide emissions from peatlands to permafrost thawing in Xiaoxing’an Mountains, Northeast China. Atmosphere 2021, 12, 222. [Google Scholar] [CrossRef]
- Guo, Y.; Song, C.; Wang, L.; Tan, W.; Wang, X.; Cui, Q.; Wan, Z. Concentrations, sources, and export of dissolved CH4 and CO2 in rivers of the permafrost wetlands, northeast China. Ecol. Eng. 2016, 90, 491–497. [Google Scholar] [CrossRef]
- Wiesenburg, D.; Guinasso, N. Equilibrium solubilities of methane, carbon monoxide, and hydrogen in water and sea water. J. Chem. Eng. Data 2002, 24, 356. [Google Scholar] [CrossRef]
- Wanninkhof, R. Relationship between wind speed and gas exchange over the ocean. J. Geophys. Res. Ocean. 1992, 97, 7373–7382. [Google Scholar] [CrossRef]
- Yan, F.; Sillanpää, M.; Kang, S.; Aho, K.; Qu, B.; Wei, D.; Li, X.; Li, C.; Raymond, P. Lakes on the Tibetan Plateau as conduits of greenhouse gases to the atmosphere. J. Geophys. Res. Biogeosci. 2018, 123, 2091–2103. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, X.; Liu, S.; Zhang, S.; Li, S.; Wang, J.; Wang, G.; Gao, H.; Zhang, Z.; Wang, Q.; et al. Significant methane ebullition from alpine permafrost rivers on the East Qinghai–Tibet Plateau. Nat. Geosci. 2020, 13, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Du, Z.; Wei, Z.; Xu, Q.; Feng, Y.; Lin, P.; Lin, J.; Chen, S.; Qiao, Y.; Shi, J.; et al. High methane emissions from thermokarst lakes on the Tibetan Plateau are largely attributed to ebullition fluxes. Sci. Total Environ. 2021, 801, 149692. [Google Scholar] [CrossRef] [PubMed]
- McEnroe, N.A.; Roulet, N.T.; Moore, T.R.; Garneau, M. Do pool surface area and depth control CO2 and CH4 fluxes from an ombrotrophic raised bog, James Bay, Canada? J. Geophys. Res. Biogeosci. 2009, 114. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Wu, Y.; Chen, H.; He, Y.; Wu, N. Intense methane ebullition from open water area of a shallow peatland lake on the eastern Tibetan Plateau. Sci. Total Environ. 2016, 542, 57–64. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; p. 1006. [Google Scholar]
- Ojala, A.; Bellido, J.L.; Tulonen, T.; Kankaala, P.; Huotari, J. Carbon gas fluxes from a brown-water and a clear-water lake in the boreal zone during a summer with extreme rain events. Limnol. Oceanogr. 2011, 56, 61–76. [Google Scholar] [CrossRef]
- Wik, M.; Crill, P.M.; Varner, R.K.; Bastviken, D. Multiyear measurements of ebullitive methane flux from three subarctic lakes. J. Geophys. Res. Biogeosci. 2013, 118, 1307–1321. [Google Scholar] [CrossRef] [Green Version]
- Yvon-Durocher, G.; Allen, A.P.; Bastviken, D.; Conrad, R.; Gudasz, C.; St-Pierre, A.; Thanh-Duc, N.; del Giorgio, P.A. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 2014, 507, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Duc, N.T.; Crill, P.; Bastviken, D. Implications of temperature and sediment characteristics on methane formation and oxidation in lake sediments. Biogeochemistry 2010, 100, 185–196. [Google Scholar] [CrossRef]
- Natchimuthu, S.; Panneer Selvam, B.; Bastviken, D. Influence of weather variables on methane and carbon dioxide flux from a shallow pond. Biogeochemistry 2014, 119, 403–413. [Google Scholar] [CrossRef]
- Burger, M.; Berger, S.; Spangenberg, I.; Blodau, C. Summer fluxes of methane and carbon dioxide from a pond and floating mat in a continental Canadian peatland. Biogeosciences 2016, 13, 3777–3791. [Google Scholar] [CrossRef] [Green Version]
- Baron, A.A.P.; Dyck, L.T.; Amjad, H.; Bragg, J.; Kroft, E.; Newson, J.; Oleson, K.; Casson, N.J.; North, R.L.; Venkiteswaran, J.J.; et al. Differences in ebullitive methane release from small, shallow ponds present challenges for scaling. Sci. Total Environ. 2022, 802, 149685. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M.; Audet, J.; Bastviken, D.; Cook, S.; Evans, C.D.; Grinham, A.; Holgerson, M.A.; Högbom, L.; Pickard, A.E.; Zieliński, P.; et al. Small artificial waterbodies are widespread and persistent emitters of methane and carbon dioxide. Glob. Change Biol. 2021, 27, 5109–5123. [Google Scholar] [CrossRef]
- Casper, P.; Maberly, S.C.; Hall, G.H.; Finlay, B.J. Fluxes of methane and carbon dioxide from a small productive lake to the atmosphere. Biogeochemistry 2000, 49, 1–19. [Google Scholar] [CrossRef]
- Boereboom, T.; Depoorter, M.; Coppens, S.; Tison, J.-L. Gas properties of winter lake ice in Northern Sweden. Biogeosci. Discuss. 2011, 9, 827–838. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, H. Spatiotemporal distribution patterns of dissolved methane in lakes: How accurate are the current estimations of the diffusive flux path? Geophys. Res. Lett. 2013, 40, 2779–2784. [Google Scholar] [CrossRef]
- Bergman, I.; Klarqvist, M.; Nilsson, M. Seasonal variation in rates of methane production from peat of various botanical origins. FEMS Microbiol. Ecol. 2000, 33, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.N.; Kulshreshtha, K.; Agnihotri, S. Seasonal dynamics of methane emission from wetlands. Chemosphere—Glob. Change Sci. 2000, 2, 39–46. [Google Scholar] [CrossRef]
- Korrensalo, A.; Mammarella, I.; Alekseychik, P.; Vesala, T.; Tuittila, E.S. Plant mediated methane efflux from a boreal peatland complex. Plant Soil 2021, 471, 375–392. [Google Scholar] [CrossRef]
- Sun, X.; Song, C.; Guo, Y.; Wang, X.; Yang, G.; Li, Y.; Mao, R.; Yongzheng, L. Effect of plants on methane emissions from a temperate marsh in different seasons. Atmos. Environ. 2012, 60, 277–282. [Google Scholar] [CrossRef]
- Turner, J.C.; Moorberg, C.J.; Wong, A.; Shea, K.; Waldrop, M.P.; Turetsky, M.R.; Neumann, R.B. Getting to the root of plant-mediated methane emissions and oxidation in a thermokarst bog. J. Geophys. Res. Biogeosci. 2020, 125, e2020JG005825. [Google Scholar] [CrossRef]
- Kao-Kniffin, J.; Freyre, D.S.; Balser, T.C. Methane dynamics across wetland plant species. Aquat. Bot. 2010, 93, 107–113. [Google Scholar] [CrossRef]
- Raghoebarsing, A.A.; Smolders, A.J.P.; Schmid, M.C.; Rijpstra, W.I.C.; Wolters-Arts, M.; Derksen, J.; Jetten, M.S.M.; Schouten, S.; Sinninghe Damsté, J.S.; Lamers, L.P.M.; et al. Methanotrophic symbionts provide carbon for photosynthesis in peat bogs. Nature 2005, 436, 1153–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kip, N.; van Winden, J.F.; Pan, Y.; Bodrossy, L.; Reichart, G.-J.; Smolders, A.J.P.; Jetten, M.S.M.; Damsté, J.S.S.; Op den Camp, H.J.M. Global prevalence of methane oxidation by symbiotic bacteria in peat-moss ecosystems. Nat. Geosci. 2010, 3, 617–621. [Google Scholar] [CrossRef]
- Winden, J.; Reichart, G.-J.; McNamara, N.; Benthien, A.; Sinninghe-Damste, J. Temperature-induced increase in methane release from peat bogs: A mesocosm experiment. PLoS ONE 2012, 7, e39614. [Google Scholar] [CrossRef] [Green Version]
- Gudasz, C.; Bastviken, D.; Steger, K.; Premke, K.; Sobek, S.; Tranvik, L.J. Temperature-controlled organic carbon mineralization in lake sediments. Nature 2010, 466, 478–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marotta, H.; Pinho, L.; Gudasz, C.; Bastviken, D.; Tranvik, L.J.; Enrich-Prast, A. Greenhouse gas production in low-latitude lake sediments responds strongly to warming. Nat. Clim. Change 2014, 4, 467–470. [Google Scholar] [CrossRef]
- Åberg, J.; Jansson, M.; Jonsson, A. Importance of water temperature and thermal stratification dynamics for temporal variation of surface water CO2 in a boreal lake. J. Geophys. Res. Biogeosci. 2010, 115. [Google Scholar] [CrossRef]
- Rantakari, M.; Kortelainen, P. Interannual variation and climatic regulation of the CO2 emission from large boreal lakes. Glob. Change Biol. 2005, 11, 1368–1380. [Google Scholar] [CrossRef]
- Ask, J.; Karlsson, J.; Jansson, M. Net ecosystem production in clear-water and brown-water lakes. Glob. Biogeochem. Cycles 2012, 26. [Google Scholar] [CrossRef]
- Shirokova, L.S.; Payandi-Rolland, D.; Lim, A.G.; Manasypov, R.M.; Allen, J.; Rols, J.L.; Bénézeth, P.; Karlsson, J.; Pokrovsky, O.S. Diel cycles of carbon, nutrient and metal in humic lakes of permafrost peatlands. Sci. Total Environ. 2020, 737, 139671. [Google Scholar] [CrossRef] [PubMed]
- Jongejans, L.; Liebner, S.; Knoblauch, C.; Mangelsdorf, K.; Ulrich, M.; Grosse, G.; Tanski, G.; Fedorov, A.; Konstantinov, P.; Windirsch, T.; et al. Greenhouse gas production and lipid biomarker distribution in Yedoma and Alas thermokarst lake sediments in Eastern Siberia. Glob. Change Biol. 2021, 27, 2822–2839. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, M.; Tranvik, L.; Natchimuthu, S.; Koehler, B. Photochemical mineralisation in a boreal brown water lake: Considerable temporal variability and minor contribution to carbon dioxide production. Biogeosciences 2016, 13, 3931–3943. [Google Scholar] [CrossRef] [Green Version]
- Juutinen, S.; Väliranta, M.; Kuutti, V.; Laine, A.M.; Virtanen, T.; Seppä, H.; Weckström, J.; Tuittila, E.S. Short-term and long-term carbon dynamics in a northern peatland-stream-lake continuum: A catchment approach. J. Geophys. Res. Biogeosci. 2013, 118, 171–183. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, X.; Duan, H.; Qi, T.; Qin, B.; Lee, X.; Hu, Z.; Wang, W.; Xiao, W.; Zhang, M. Eutrophic Lake Taihu as a significant CO2 source during 2000–2015. Water Res. 2020, 170, 115331. [Google Scholar] [CrossRef] [PubMed]
- Van de Bogert, M.C.; Bade, D.L.; Carpenter, S.R.; Cole, J.J.; Pace, M.L.; Hanson, P.C.; Langman, O.C. Spatial heterogeneity strongly affects estimates of ecosystem metabolism in two north temperate lakes. Limnol. Oceanogr. 2012, 57, 1689–1700. [Google Scholar] [CrossRef] [Green Version]
- Woszczyk, M.; Schubert, C.J. Greenhouse gas emissions from Baltic coastal lakes. Sci. Total Environ. 2021, 755, 143500. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; Smolenski, R.L.; Nietch, C.T.; Townsend-Small, A.; Elovitz, M.S.; Schubauer-Berigan, J.P. Denitrification alternates between a source and sink of nitrous oxide in the hypolimnion of a thermally stratified reservoir. Limnol. Oceanogr. 2014, 59, 495–506. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; Arango, C.P.; Hamilton, S.K.; Tank, J.L. The production and emission of nitrous oxide from headwater streams in the Midwestern United States. Glob. Change Biol. 2008, 14, 878–894. [Google Scholar] [CrossRef]
- Webb, J.; Hayes, N.; Simpson, G.; Leavitt, P.; Baulch, H.; Finlay, K. Widespread nitrous oxide undersaturation in farm waterbodies creates an unexpected greenhouse gas sink. Proc. Natl. Acad. Sci. USA 2019, 116, 9814–9819. [Google Scholar] [CrossRef] [Green Version]
- Sturm, K.; Yuan, Z.; Gibbes, B.; Werner, U.; Grinham, A. Methane and nitrous oxide sources and emissions in a subtropical freshwater reservoir, South East Queensland, Australia. Biogeosciences 2014, 11, 5245–5258. [Google Scholar] [CrossRef] [Green Version]
- Barnes, J.; Upstill-Goddard, R.C. The denitrification paradox: The role of O2 in sediment N2O production. Estuar. Coast. Shelf Sci. 2018, 200, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, L.; Yao, X.; Xue, B.; Yan, W. Dissolved nitrous oxide and emission relating to denitrification across the Poyang Lake aquatic continuum. J. Environ. Sci. 2016, 52, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, J.T.; Väisänen, T.S.; Heikkinen, M.; Hellsten, S.; Nykänen, H.; Nenonen, O.; Martikainen, P.J. Exchange of CO2, CH4 and N2O between the atmosphere and two northern boreal ponds with catchments dominated by peatlands or forests. Plant Soil 2002, 242, 137–146. [Google Scholar] [CrossRef]
- Miao, Y.; Huang, J.; Duan, H.; Meng, H.; Wang, Z.; Qi, T.; Wu, Q.L. Spatial and seasonal variability of nitrous oxide in a large freshwater lake in the lower reaches of the Yangtze River, China. Sci. Total Environ. 2020, 721, 137716. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, X.; Song, K.; Yeerken, S.; Deng, M.; Li, L.; Riya, S.; Wang, Q.; Terada, A. Nonlinear pattern and algal dual-impact in N2O emission with increasing trophic levels in shallow lakes. Water Res. 2021, 203, 117489. [Google Scholar] [CrossRef]
- Kortelainen, P.; Rantakari, M.; Huttunen, J.T.; Mattsson, T.; Alm, J.; Juutinen, S.; Larmola, T.; Silvola, J.; Martikainen, P.J. Sediment respiration and lake trophic state are important predictors of large CO2 evasion from small boreal lakes. Glob. Change Biol. 2006, 12, 1554–1567. [Google Scholar] [CrossRef]
- Pi, X.; Luo, Q.; Feng, L.; Xu, Y.; Tang, J.; Liang, X.; Ma, E.; Cheng, R.; Fensholt, R.; Brandt, M.; et al. Mapping global lake dynamics reveals the emerging roles of small lakes. Nat. Commun. 2022, 13, 5777. [Google Scholar] [CrossRef]







| Site | Water Table (cm) | Plant Biomass (g DWm−2) | Plant Height (cm) | Maximum Depth of Active Layer (cm) |
|---|---|---|---|---|
| Pelagic zone | 116.00 ± 2.56 | ND | ND | 105.67 ± 0.65 |
| Littoral zone | 21.4 ± 25.1 | 110.43 ± 21.58 | 33.50 ± 6.50 | 96.16 ± 0.84 |
| Bog | −12.25 ± 0.68 | 287.76 ± 5.12 | 135 ± 14.98 | 80.00 ± 2.42 |
| Site | TOC (g kg−1) | TN (g kg−1) | pH | C/N |
|---|---|---|---|---|
| Pelagic zone | 297.00 ± 41.41 c | 36.63 ± 0.73 a | 4.15 ± 0.03 c | 8.11 |
| Littoral zone | 350.67 ± 12.67 b | 27.62 ± 0.02 b | 4.38 ± 0.39 b | 12.7 |
| Bog | 527.33 ± 10.23 a | 32.85 ± 1.58 ab | 4.57 ± 0.04 a | 16.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; Chen, X.; Wang, X.; Sun, X. Fine-Scale Assessment of Greenhouse Gases Fluxes from a Boreal Peatland Pond. Water 2023, 15, 307. https://doi.org/10.3390/w15020307
Xue J, Chen X, Wang X, Sun X. Fine-Scale Assessment of Greenhouse Gases Fluxes from a Boreal Peatland Pond. Water. 2023; 15(2):307. https://doi.org/10.3390/w15020307
Chicago/Turabian StyleXue, Jing, Xinan Chen, Xianwei Wang, and Xiaoxin Sun. 2023. "Fine-Scale Assessment of Greenhouse Gases Fluxes from a Boreal Peatland Pond" Water 15, no. 2: 307. https://doi.org/10.3390/w15020307