Development of Fouling-Control Strategy for Ceramic Membrane Bioreactor Applied in Partial Nitrification Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthetic Wastewater and Seeding Sludge
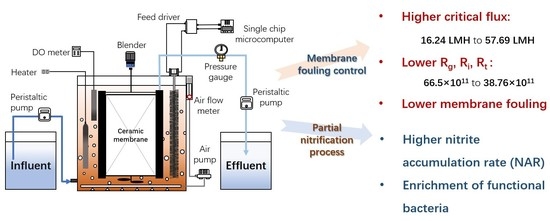
2.2. Construction and Operation of Membrane Bioreactor
2.3. Chemical Cleaning Method
2.4. Analytical Methods
2.5. Data Analysis
2.6. Microbial Community Analyses
3. Results and Discussion
3.1. In Situ Membrane Fouling Control
3.2. Partial Nitrification Start-Up and Stabilization
3.3. Microbial Community Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, L.; Ji, M.; Wang, F.; Wang, S.; Qin, G. Insight into the influence of microbial aggregate types on nitrogen removal performance and microbial community in the anammox process—A review and meta-analysis. Sci. Total Environ. 2020, 714, 136571. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ji, Q.; Ding, S.; Zheng, P. The morphological and settling properties of ANAMMOX granular sludge in high-rate reactors. Bioresour. Technol. 2013, 143, 592–597. [Google Scholar] [CrossRef] [PubMed]
- van de Graaf, A.A.; de Bruijn, P.; Robertson, L.A.; Jetten, M.S.M.; Kuenen, J.G. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 1996, 142, 2187–2196. [Google Scholar] [CrossRef] [Green Version]
- Chatzisymeon, E. Application of Biological and Chemical Processes to Wastewater Treatment. Water 2021, 13, 1781. [Google Scholar] [CrossRef]
- Li, X.; Xia, C.; Sun, Y.; Ding, W.; Qin, H. Characteristics of Nitrifying and Denitrifying Microbes in the Bioretention Cell with Submerged Zone during a Dry Period. Water 2022, 14, 3503. [Google Scholar] [CrossRef]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Akaboci, T.R.V.; Gich, F.; Ruscalleda, M.; Balaguer, M.D.; Colprim, J. Effects of extremely low bulk liquid DO on autotrophic nitrogen removal performance and NOB suppression in side- and mainstream one-stage PNA. J. Chem. Technol. Biotechnol. 2018, 93, 2931–2941. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, F.; Liu, S.; Bao, H.; Hu, S.; Furukawa, K. Feasibility of a membrane-aerated biofilm reactor to achieve single-stage autotrophic nitrogen removal based on Anammox. Chemosphere 2007, 69, 776–784. [Google Scholar] [CrossRef]
- van der Star, W.R.L.; Miclea, A.I.; van Dongen, U.G.J.M.; Muyzer, G.; Picioreanu, C.; van Loosdrecht, M.C.M. The membrane bioreactor: A novel tool to grow anammox bacteria as free cells. Biotechnol. Bioeng. 2008, 101, 286–294. [Google Scholar] [CrossRef]
- Huang, X.; Urata, K.; Wei, Q.; Yamashita, Y.; Hama, T.; Kawagoshi, Y. Fast start-up of partial nitritation as pre-treatment for anammox in membrane bioreactor. Biochem. Eng. J. 2016, 105, 371–378. [Google Scholar] [CrossRef]
- Liang, Z.; Han, Z.; Yang, S.; Liang, X.; Du, P.; Liu, G.; Yang, Y. A control strategy of partial nitritation in a fixed bed bioflim reactor. Bioresour. Technol. 2011, 102, 710–715. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, J. Control factors of partial nitritation for landfill leachate treatment. J. Environ. Sci. 2007, 19, 523–529. [Google Scholar] [CrossRef]
- Awata, T.; Goto, Y.; Kuratsuka, H.; Aoi, Y.; Ozaki, N.; Ohashi, A.; Kindaichi, T. Reactor performance and microbial community structure of single-stage partial nitritation anammox membrane bioreactors inoculated with Brocadia and Scalindua enrichment cultures. Biochem. Eng. J. 2021, 170, 107991. [Google Scholar] [CrossRef]
- Mao, X.; Myavagh, P.H.; Lotfikatouli, S.; Hsiao, B.S.; Walker, H.W. Membrane Bioreactors for Nitrogen Removal from Wastewater: A Review. J. Environ. Eng. 2020, 146, 03120002. [Google Scholar] [CrossRef]
- Gu, Q.; Ng, T.C.A.; Bao, Y.; Ng, H.Y.; Tan, S.C.; Wang, J. Developing better ceramic membranes for water and wastewater Treatment: Where microstructure integrates with chemistry and functionalities. Chem. Eng. J. 2022, 428, 130456. [Google Scholar] [CrossRef]
- Kayvani Fard, A.; McKay, G.; Buekenhoudt, A.; Al Sulaiti, H.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Nywening, J.-P.; Zhou, H. Influence of filtration conditions on membrane fouling and scouring aeration effectiveness in submerged membrane bioreactors to treat municipal wastewater. Water Res. 2009, 43, 3548–3558. [Google Scholar] [CrossRef]
- van den Brink, P.; Vergeldt, F.; Van As, H.; Zwijnenburg, A.; Temmink, H.; van Loosdrecht, M.C.M. Potential of mechanical cleaning of membranes from a membrane bioreactor. J. Membr. Sci. 2013, 429, 259–267. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Rout, P.R.; Aslam, M.; Fuwad, A.; Choi, Y.; Banu, J.R.; Park, J.H.; Kumar, G. A brief review of anaerobic membrane bioreactors emphasizing recent advancements, fouling issues and future perspectives. J. Environ. Manag. 2020, 270, 110909. [Google Scholar] [CrossRef]
- Fan, F.; Zhou, H. Interrelated effects of aeration and mixed liquor fractions on membrane fouling for submerged membrane bioreactor processes in wastewater treatment. Environ. Sci. Technol. 2007, 41, 2523–2528. [Google Scholar] [CrossRef] [PubMed]
- APHA; AWWA; WEF. Standard Methods for Examination of Water and Wastewater, 20th ed.; Ameican Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Soliman, M.; Eldyasti, A. Development of partial nitrification as a first step of nitrite shunt process in a Sequential Batch Reactor (SBR) using Ammonium Oxidizing Bacteria (AOB) controlled by mixing regime. Bioresour. Technol. 2016, 221, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.S.; Zhu, X.L.; Wu, J.; Tian, Z.; Bai, Y.H.; Huang, B.C.; Jin, R.C. Deciphering the microbial and genetic responses of anammox biogranules to the single and joint stress of zinc and tetracycline. Environ. Int. 2019, 132, 105097. [Google Scholar] [CrossRef] [PubMed]
- Kunacheva, C.; Soh, Y.N.A.; Stuckey, D.C. Identification of soluble microbial products (SMPs) from the fermentation and methanogenic phases of anaerobic digestion. Sci. Total Environ. 2020, 698, 134177. [Google Scholar] [CrossRef] [PubMed]
- Defrance, L.; Jaffrin, M.Y. Comparison between ®ltrations at ®xed transmembrane pressure and ®xed permeate ¯ux: Application to a membrane bioreactor used for wastewater treatment. J. Membr. Sci. 1999, 8, 203–210. [Google Scholar] [CrossRef]
- Field, R.W.; Wu, D.; Howell, J.A.; Gupta, B.B. Critical flux concept for microfiltration fouling. J. Membr. Sci. 1995, 100, 259–272. [Google Scholar] [CrossRef]
- Aghapour Aktij, S.; Taghipour, A.; Rahimpour, A.; Mollahosseini, A.; Tiraferri, A. A critical review on ultrasonic-assisted fouling control and cleaning of fouled membranes. Ultrasonics 2020, 108, 106228. [Google Scholar] [CrossRef]
- Ninomiya, Y.; Kimura, K.; Sato, T.; Kakuda, T.; Kaneda, M.; Hafuka, A.; Tsuchiya, T. High-flux operation of MBRs with ceramic flat-sheet membranes made possible by intensive membrane cleaning: Tests with real domestic wastewater under low-temperature conditions. Water Res. 2020, 181, 115881. [Google Scholar] [CrossRef]
- Braak, E.; Alliet, M.; Schetrite, S.; Albasi, C. Aeration and hydrodynamics in submerged membrane bioreactors. J. Membr. Sci. 2011, 379, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Pourabdollah, M.; Torkian, A.; Hashemian, S.J.; Bakhshi, B. A triple fouling layers perspective on evaluation of membrane fouling under different scenarios of membrane bioreactor operation. J. Environ. Health Sci. Engineer. 2014, 12, 91. [Google Scholar] [CrossRef]
- Qian, G.; Xie, C.; Teng, H.; Zhang, Y.; Zhao, H.; Zhou, J. Effect of aeration on membrane fouling and critical flux in microfiltration tubular membranes. Chin. J. Environ. Eng. 2017, 11, 4542–4548. [Google Scholar]
- Daly, S.; Casey, E.; Semião, A.J.C. Osmotic backwashing of forward osmosis membranes to detach adhered bacteria and mitigate biofouling. J. Membr. Sci. 2021, 620, 118838. [Google Scholar] [CrossRef]
- Thejani Nilusha, R.; Wang, T.; Wang, H.; Yu, D.; Zhang, J.; Wei, Y. Optimization of In Situ Backwashing Frequency for Stable Operation of Anaerobic Ceramic Membrane Bioreactor. Processes 2020, 8, 545. [Google Scholar] [CrossRef]
- Zhou, Z.; Qi, M.; Wang, H. Achieving Partial Nitrification via Intermittent Aeration in SBR and Short-Term Effects of Different C/N Ratios on Reactor Performance and Microbial Community Structure. Water 2020, 12, 3485. [Google Scholar] [CrossRef]
- van Dongen, U.; Jetten, M.S.M.; van Loosdrecht, M.C.M. The SHARON((R))-Anammox((R)) process for treatment of ammonium rich wastewater. Water Sci. Technol. 2001, 44, 153–160. [Google Scholar] [CrossRef]
- Li, J.; Qiang, Z.; Yu, D.; Wang, D.; Zhang, P.; Li, Y. Performance and microbial community of simultaneous anammox and denitrification (SAD) process in a sequencing batch reactor. Bioresour. Technol. 2016, 218, 1064–1072. [Google Scholar] [CrossRef]
- Chen, W.; Dai, X.; Cao, D.; Hu, X.; Liu, W.; Yang, D. Characterization of a Microbial Community in an Anammox Process Using Stored Anammox Sludge. Water 2017, 9, 829. [Google Scholar] [CrossRef] [Green Version]
- Soler-Jofra, A.; Schmidtchen, L.; Olmo, L.; van Loosdrecht, M.C.M.; Pérez, J. Short and long term continuous hydroxylamine feeding in a granular sludge partial nitritation reactor. Water Res. 2022, 209, 117945. [Google Scholar] [CrossRef]
- Liu, A.; Zhao, K.-L.; Liu, H.; Huang, L.; Ni, R.; Chen, Y.-Z. Short-cut Nitrification Start-up and Optimization of Operating Conditions Under Different Control Strategies. Huan Jing Ke Xue 2019, 40, 4569–4577. [Google Scholar]
- Wang, H.; Wang, Y.; Zhang, J.; Sui, Q.; Hu, D.; Zuo, F.; Wei, Y. Is Anoxic Operation Effective to Control Nitrate Build-Up and Sludge Loss for the Combined Partial Nitritation and Anammox (CPNA) Process? Processes 2020, 8, 1053. [Google Scholar] [CrossRef]
- Prakasam, T.; Loehr, R. Microbial nitrification and denitrification in concentrated wastes. Water Res. 1972, 6, 859–869. [Google Scholar] [CrossRef]
- Van Hulle, S.W.; Volcke, E.I.; Teruel, J.L.; Donckels, B.; van Loosdrecht, M.C.; Vanrolleghem, P.A. Influence of temperature and pH on the kinetics of the Sharon nitritation process. J. Chem. Technol. Biotechnol. 2007, 82, 471–480. [Google Scholar] [CrossRef]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of Nitrification by Ammonia and Nitrous Acid. J. Water Pollut. Control. Fed. 1976, 48, 835–852. [Google Scholar]
- Vadivelu, V.M.; Keller, J.; Yuan, Z. Free ammonia and free nitrous acid inhibition on the anabolic and catabolic processes of Nitrosomonas and Nitrobacter. Water Sci. Technol. 2007, 56, 89–97. [Google Scholar] [CrossRef]
- Vadivelu, V.M.; Yuan, Z.; Fux, C.; Keller, J. The inhibitory effects of free nitrous acid on the energy generation and growth processes of an enriched nitrobacter culture. Environ. Sci. Technol. 2006, 40, 4442–4448. [Google Scholar] [CrossRef]
- Ge, S.; Peng, Y.; Qiu, S.; Zhu, A.; Ren, N. Complete nitrogen removal from municipal wastewater via partial nitrification by appropriately alternating anoxic/aerobic conditions in a continuous plug-flow step feed process. Water Res. 2014, 55, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Yang, S.; Zhang, Q.; Li, X.; Zhang, L.; Peng, Y. Rapid achieving partial nitrification in domestic wastewater: Controlling aeration time to selectively enrich ammonium oxidizing bacteria (AOB) after simultaneously eliminating AOB and nitrite oxidizing bacteria (NOB). Bioresour. Technol. 2021, 328, 124810. [Google Scholar] [CrossRef]
- Tian, W.-D.; An, K.-J.; Ma, C.; Han, X. Partial nitritation for subsequent Anammox to treat high-ammonium leachate. Environ. Technol. 2013, 34, 1063–1068. [Google Scholar] [CrossRef]
- Vadivelu, V.M.; Keller, J.; Yuan, Z. Effect of free ammonia on the respiration and growth processes of an enriched Nitrobacter culture. Water Res. 2007, 41, 826–834. [Google Scholar] [CrossRef]
- Shen, L.; Yao, Y.; Meng, F. Reactor performance and microbial ecology of a nitritation membrane bioreactor. J. Membr. Sci. 2014, 462, 139–146. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, P.; Shen, Y.-L.; Lü, G.; Xu, Y.-Z.; Samwine, T. Fast Start-up of Shortcut Nitrification in a CSTR and an MBR. Huan Jing Ke Xue 2017, 38, 3399–3405. [Google Scholar] [PubMed]
- Wu, P.; Chen, Y.; Zhang, T.; Shen, Y.-L.; Xu, Y.-Z. Microbial Community Characteristics of Shortcut Nitrification Start-up in Different MBR-Inoculated Sludges. Huan Jing Ke Xue 2018, 39, 4636–4643. [Google Scholar] [PubMed]
- Wang, Y.; Bu, C.N.; Kang, Q.; Ahmad, H.A.; Zhang, J.; Gao, B.; Ni, S.Q. Autoclaved sludge as the ideal seed to culture anammox bacteria: Reactor performance and microbial community diversity. Bioresour. Technol. 2017, 244, 391–399. [Google Scholar] [CrossRef]






| Phase | Time (d) | Inf. NH4+-N (mg/L) | HRT (h) | NLR (kg N/m3/d) |
|---|---|---|---|---|
| I | 1–10 | 100 | 24 | 0.1 |
| II | 11–22 | 200 | 24 | 0.2 |
| III | 23–106 | 400 | 24 | 0.4 |
| 107–136 | 200 | 12 | 0.4 | |
| IV | 137–156 | 300 | 12 | 0.6 |
| Total Resistance (Rt) | Membrane Resistance (Rm) | Concentration Polarization Resistance (Rc) | Gel Layer Resistance (Rg) | Inherent Resistance (Ri) | |
|---|---|---|---|---|---|
| Without in-situ cleaning (1011/m) | 66.50 | 8.31 | 20.19 | 23.72 | 14.28 |
| Percentage (%) | 100.00 | 12.50 | 30.36 | 35.67 | 21.47 |
| With membrane cleaning devices (1011/m) | 38.76 | 8.31 | 17.39 | 5.58 | 7.48 |
| Percentage (%) | 100.00 | 21.44 | 44.86 | 14.40 | 19.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Wang, R.; Zuo, W.; Peng, Y.; An, D.; Zhang, L.; Ge, Z. Development of Fouling-Control Strategy for Ceramic Membrane Bioreactor Applied in Partial Nitrification Process. Water 2023, 15, 444. https://doi.org/10.3390/w15030444
Li B, Wang R, Zuo W, Peng Y, An D, Zhang L, Ge Z. Development of Fouling-Control Strategy for Ceramic Membrane Bioreactor Applied in Partial Nitrification Process. Water. 2023; 15(3):444. https://doi.org/10.3390/w15030444
Chicago/Turabian StyleLi, Bingxin, Ruochen Wang, Weiwei Zuo, Yi Peng, Dong An, Liang Zhang, and Zheng Ge. 2023. "Development of Fouling-Control Strategy for Ceramic Membrane Bioreactor Applied in Partial Nitrification Process" Water 15, no. 3: 444. https://doi.org/10.3390/w15030444