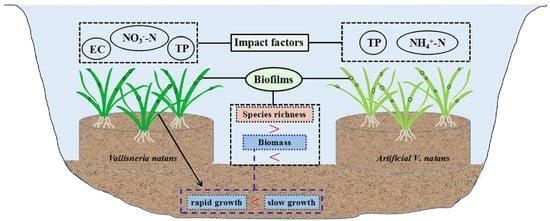
Dynamic Characteristics of Periphytic Algae Communities on Different Substrates and the Host Response in Subtropical-Urban-Landscape Lakes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Experimental Setup
2.3. Periphytic Algae Sampling and Treatment
2.4. Determination of Malondialdehyde (MDA) Content
2.5. Determination of Physio-Chemical Parameters of Water
2.6. Statistical Analyses
3. Results
3.1. Physio-Chemical Characteristics
3.2. Periphytic-Algae-Community Compositions on V. natans and Artificial V. natans
3.2.1. Periphytic-Algae-Community Species Richness
3.2.2. Host Preferences of Periphytic Algae on Different Substrates
3.2.3. Dominant Periphytic-Algae Community Taxa
3.3. Periphytic Algae Biomass on V. natans and Artificial V. natans
3.4. Diversity Index and Similarity Coefficient of Periphytic Algae on V. natans and Artificial V. natans
3.5. Relationships between Environmental Variables and Periphytic Algae
3.6. Changes in MDA Content of V. natans
4. Discussion
4.1. Periphytic Algae Community on Different Substrate and Time
4.2. Relationships between the Periphytic Algae Community and Environmental Parameters
4.3. Response of V. natans to Periphytic Algae
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weerasinghe, V.; Handapangoda, K. Surface water quality analysis of an urban lake; East Beira, Colombo, Sri Lanka. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100249. [Google Scholar] [CrossRef]
- Mao, X.; Wei, X.; Jin, X.; Tao, Y.; Zhang, Z.; Wang, W. Monitoring urban wetlands restoration in Qinghai Plateau: Integrated performance from ecological characters, ecological processes to ecosystem services. Ecol. Indic. 2019, 101, 623–631. [Google Scholar] [CrossRef]
- Phillips, G.; Willby, N.; Moss, B. Submerged macrophyte decline in shallow lakes: What have we learnt in the last forty years? Aquat. Bot. 2016, 135, 37–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeppesen, E.; Liu, X.; Qin, B.; Shi, K.; Zhou, Y.; Thomaz, S.M.; Deng, J. Global loss of aquatic vegetation in lakes. Earth-Sci. Rev. 2017, 173, 259–265. [Google Scholar] [CrossRef]
- Paerl, H.W.; Havens, K.E.; Hall, N.S.; Otten, T.G.; Zhu, M.; Xu, H.; Zhu, G.; Qin, B. Mitigating a global expansion of toxic cyanobacterial blooms: Confounding effects and challenges posed by climate change. Mar. Freshw. Res. 2020, 71, 579. [Google Scholar] [CrossRef]
- Celewicz-Gołdyn, S.; Kuczyńska-Kippen, N. Ecological value of macrophyte cover in creating habitat for microalgae (diatoms) and zooplankton (rotifers and crustaceans) in small field and forest water bodies. PLoS ONE 2017, 12, e0177317. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, X.; Cao, T.; Ni, L.; Xie, P. Reconstructing clear water state and submersed vegetation on behalf of repeated flocculation with modified soil in an in situ mesocosm experiment in Lake Taihu. Sci. Total Environ. 2018, 625, 1433–1445. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, S.; Peng, X.; Liu, B.; Zhang, Y.; Zhou, Q.; Wu, Z. The Response of Regeneration Ability of Myriophyllum spicatum Apical Fragments to Decaying Cladophora oligoclona. Water 2019, 11, 1014. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, H.; Zhang, J.; Yu, J.; Xie, P.; Chen, J. Physiological differences between free-floating and periphytic filamentous algae, and specific submerged macrophytes induce proliferation of filamentous algae: A novel implication for lake restoration. Chemosphere 2020, 239, 124702. [Google Scholar] [CrossRef]
- Rodrigo, M.A.; Rojo, C.; Alonso-Guillén, J.L.; Vera, P. Restoration of two small Mediterranean lagoons: The dynamics of submerged macrophytes and factors that affect the success of revegetation. Ecol. Eng. 2013, 54, 1–15. [Google Scholar] [CrossRef]
- Peng, X.; Yi, K.; Lin, Q.; Zhang, L.; Zhang, Y.; Liu, B.; Wu, Z. Annual changes in periphyton communities and their diatom indicator species, in the littoral zone of a subtropical urban lake restored by submerged plants. Ecol. Eng. 2020, 155, 105958. [Google Scholar] [CrossRef]
- Mormul, R.P.; Ahlgren, J.; Brönmark, C. Snails have stronger indirect positive effects on submerged macrophyte growth attributes than zooplankton. Hydrobiologia 2018, 807, 165–173. [Google Scholar] [CrossRef]
- Dong, B.; Han, R.; Wang, G.; Cao, X. O2, pH, and Redox Potential Microprofiles around Potamogeton malaianus Measured Using Microsensors. PLoS ONE 2014, 9, e101825. [Google Scholar] [CrossRef]
- Guan, J.; Jacoby, C.A.; Frazer, T.K. Light attenuation by periphyton on Vallisneria americana. Ecol. Indic. 2020, 116, 106498. [Google Scholar] [CrossRef]
- Letáková, M.; Fránková, M.; Poulíčková, A. Ecology and Applications of Freshwater Epiphytic Diatoms—Review. Cryptogam. Algol. 2018, 39, 3–22. [Google Scholar] [CrossRef]
- Mohamed, Z.A.; Al Shehri, A.M. Microcystin production in epiphytic cyanobacteria on submerged macrophytes. Toxicon 2010, 55, 1346–1352. [Google Scholar] [CrossRef]
- Min, F.; Zuo, J.; Zhang, Y.; Lin, Q.; Liu, B.; Sun, J.; Zeng, L.; He, F.; Wu, Z. The Biomass and Physiological Responses of Vallisneria natans (Lour.) Hara to Epiphytic Algae and Different Nitrate-N Concentrations in the Water Column. Water 2017, 9, 863. [Google Scholar] [CrossRef]
- Wolters, J.; Reitsema, R.E.; Verdonschot, R.C.M.; Schoelynck, J.; Verdonschot, P.F.M.; Meire, P. Macrophyte-specific effects on epiphyton quality and quantity and resulting effects on grazing macroinvertebrates. Freshw. Biol. 2019, 64, 1131–1142. [Google Scholar] [CrossRef]
- Levi, P.S.; Starnawski, P.; Poulsen, B.; Baattrup-Pedersen, A.; Schramm, A.; Riis, T. Microbial community diversity and composition varies with habitat characteristics and biofilm function in macrophyte-rich streams. Oikos 2017, 126, 398–409. [Google Scholar] [CrossRef]
- dos Santos, T.R.; Ferragut, C.; de Mattos Bicudo, C.E. Does macrophyte architecture influence periphyton? Relationships among Utricularia foliosa, periphyton assemblage structure and its nutrient (C, N, P) status. Hydrobiologia 2013, 714, 71–83. [Google Scholar] [CrossRef]
- Cai, X.; Gao, G.; Tang, X.; Dong, B.; Dai, J.; Chen, D.; Song, Y. The response of epiphytic microbes to habitat and growth status of Potamogeton malaianus Miq. in Lake Taihu. J. Basic Microbiol. 2013, 53, 828–837. [Google Scholar] [CrossRef]
- Olsen, S.; Chan, F.; Li, W.; Zhao, S.; Søndergaard, M.; Jeppesen, E. Strong impact of nitrogen loading on submerged macrophytes and algae: A long-term mesocosm experiment in a shallow Chinese lake. Freshw. Biol. 2015, 60, 1525–1536. [Google Scholar] [CrossRef]
- Hu, H.J.; Wei, Y.X. The Freshwater Algae of China: Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Tunca, H.; Sevindik, T.O.; Bal, D.N.; Arabaci, S. Community structure of epiphytic algae on three different macrophytes at Acarlar floodplain forest (northern Turkey). Chin. J. Oceanol. Limnol. 2014, 32, 845–857. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, S.; Chen, D.; Liu, K.; Lu, J. Response of biofilms-leaves of two submerged macrophytes to high ammonium. Chemosphere 2018, 192, 152–160. [Google Scholar] [CrossRef]
- State EPA of China. Monitoring and Determination Methods for Water and Wastewater, 4th ed.; China Environmental Science Press: Beijing, China, 2002; pp. 243–285.
- Shan, K.; Song, L.; Chen, W.; Li, L.; Liu, L.; Wu, Y.; Jia, Y.; Zhou, Q.; Peng, L. Analysis of environmental drivers influencing interspecific variations and associations among bloom-forming cyanobacteria in large, shallow eutrophic lakes. Harmful Algae 2019, 84, 84–94. [Google Scholar] [CrossRef]
- Tarkowska-Kukuryk, M.; Pęczuła, W.; Mieczan, T. Grazing affects periphytic algal biomass in the periphyton-macrophyte relationship independently of the substrate type and nutrient status. J. Limnol. 2020, 79, 124–137. [Google Scholar] [CrossRef]
- Feng, J.; Wang, F.; Xie, S. Structure and dynamics of the periphytic algae of Jinyang Lake in Shanxi Province, North China. Acta Ecol. Sin. 2011, 31, 310–316. [Google Scholar] [CrossRef]
- Hoagland, K.D.; Roemer, S.C.; Rosowski, J.R. Colonization and community structure of two periphyton assemblages, with emphasis on the diatoms (Bacillariophyceae). Am. J. Bot. 1982, 69, 188–213. [Google Scholar] [CrossRef]
- Nian, Y.; Han, Y.Z.; Yang, Z.F. Ecological characteristics of periphyton communities on different kinds of substrates and comparison. Environ. Sci. Technol. 2009, 5, 18–21. (In Chinese) [Google Scholar]
- Toporowska, M.; Pawlik-Skowrońska, B.; Wojtal, A. Epiphytic algae on Stratiotes aloides L., Potamogeton lucens L., Ceratophyllum demersum L. and Chara spp. in a macrophyte-dominated lake. Oceanol. Hydrobiol. Stud. 2008, 37, 51–63. [Google Scholar] [CrossRef]
- Pomazkina, G.; Kravtsova, L.; Sorokovikova, E. Structure of epiphyton communities on Lake Baikal submerged macrophytes. Limnol. Rev. 2012, 12, 19–27. [Google Scholar] [CrossRef]
- Manna, S.; Ghosh, R.; Sarkar, N.S.; Roy, A. Diversity and association analysis of algal periphyton community on Hydrilla verticillata, Vallisneria spiralis and Ceratophyllum demersum. Res. J. Pharm. Biol. Chem. Sci. 2017, 8, 1232–1240. [Google Scholar]
- Hao, B.; Wu, H.; Cao, Y.; Xing, W.; Jeppesen, E.; Li, W. Comparison of periphyton communities on natural and artificial macrophytes with contrasting morphological structures. Freshw. Biol. 2017, 62, 1783–1793. [Google Scholar] [CrossRef]
- Wijewardene, L.; Wu, N.; Fohrer, N.; Riis, T. Epiphytic biofilms in freshwater and interactions with macrophytes: Current understanding and future directions. Aquat. Bot. 2022, 176, 103467. [Google Scholar] [CrossRef]
- Mulderij, G.; Mau, B.; Domis, L.N.D.S.; Smolders, A.J.P.; Van Donk, E. Interaction between the macrophyte Stratiotes aloides and filamentous algae: Does it indicate allelopathy? Aquat. Ecol. 2009, 43, 305–312. [Google Scholar] [CrossRef]
- He, D.; Zheng, J.; Ren, L.; Wu, Q.L. Substrate type and plant phenolics influence epiphytic bacterial assembly during short-term succession. Sci. Total Environ. 2021, 792, 148410. [Google Scholar] [CrossRef]
- Sultana, M.; Asaeda, T.; Manatunge, J.; Ablimit, A. Colonisation and growth of epiphytic algal communities on Potamogeton perfoliatus under two different light regimes. New Zealand J. Mar. Freshw. Res. 2004, 38, 585–594. [Google Scholar] [CrossRef]
- John, D.M.; Rindi, F. Filamentous (Nonconjugating) and Plantlike Green Algae. In Freshwater Algae of North America; Wehr, J.W., Sheath, R.G., Kociolek, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 375–427. [Google Scholar] [CrossRef]
- Song, Y.-Z.; Wang, J.-Q.; Gao, Y.-X. Effects of epiphytic algae on biomass and physiology of Myriophyllum spicatum L. with the increase of nitrogen and phosphorus availability in the water body. Environ. Sci. Pollut. Res. 2017, 24, 9548–9555. [Google Scholar] [CrossRef]
- Casartelli, M.R.; Ferragut, C. The effects of habitat complexity on periphyton biomass accumulation and taxonomic structure during colonization. Hydrobiologia 2018, 807, 233–246. [Google Scholar] [CrossRef]
- Pfeiffer, T.; Mihaljevič, M.; Spoljaric, D.; Stević, F.; Plenkovic-Moraj, A. The disturbance-driven changes of periphytic algal communities in a Danubian floodplain lake. Knowl. Manag. Aquat. Ecosyst. 2015, 416, 2. [Google Scholar] [CrossRef]
- Song, Y.; Wang, J.; Gao, Y.; Qin, B. Nitrogen incorporation by epiphytic algae via Vallisneria natans using 15N tracing in sediment with increasing nutrient availability. Aquat. Microb. Ecol. 2017, 80, 93–99. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, H.-J.; Wang, H.-Z.; Li, Y.; Liang, X.-M.; Xu, C.; Jeppesen, E. Does the responses of Vallisneria natans (Lour.) Hara to high nitrogen loading differ between the summer high-growth season and the low-growth season? Sci. Total Environ. 2017, 601–602, 1513–1521. [Google Scholar] [CrossRef]
- Adam, M.S.; Hifney, A.F.; Fawzy, M.A.; Al-Badaani, A.A. Seasonal biodiversity and ecological studies on the epiphytc microalgae communites in polluted and unpolluted aquatc ecosystem at Assiut, Egypt. Eur. J. Ecol. 2017, 3, 92–106. [Google Scholar] [CrossRef]
- McDowell, R.W.; Noble, A.; Pletnyakov, P.; Haggard, B.E.; Mosley, L.M. Global mapping of freshwater nutrient enrichment and periphyton growth potential. Sci. Rep. 2020, 10, 3568. [Google Scholar] [CrossRef]
- Song, Y.-Z.; Kong, F.-F.; Xue, Y.; Qin, B.-Q. Responses of chlorophyll and MDA of Vallisneria natans to nitrogen and phosphorus availability and epiphytic algae. J. Freshw. Ecol. 2015, 30, 85–97. [Google Scholar] [CrossRef]
- Ray, A.M.; Mebane, C.A.; Raben, F.; Irvine, K.M.; Marcarelli, A.M. Evaluation of a combined macrophyte–epiphyte bioassay for assessing nutrient enrichment in the Portneuf River, Idaho, USA. Environ. Monit. Assess. 2014, 186, 4081–4096. [Google Scholar] [CrossRef]
- Foster, R.A.; Kuypers, M.M.M.; Vagner, T.; Paerl, R.W.; Musat, N.; Zehr, J.P. Nitrogen fixation and transfer in open ocean diatom–cyanobacterial symbioses. ISME J. 2011, 5, 1484–1493. [Google Scholar] [CrossRef]
- Andrus, J.M.; Winter, D.; Scanlan, M.; Sullivan, S.; Bollman, W.; Waggoner, J.; Hosmer, A.J.; Brain, R.A. Seasonal synchronicity of algal assemblages in three Midwestern agricultural streams having varying concentrations of atrazine, nutrients, and sediment. Sci. Total Environ. 2013, 458–460, 125–139. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, S.; Lin, D.; Guo, C.; Yan, L.; Wang, S.; He, Z. Nitrogen loading affects microbes, nitrifiers and denitrifiers attached to submerged macrophyte in constructed wetlands. Sci. Total Environ. 2018, 622–623, 121–126. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Adyel, T.M.; Liu, K.; Gong, L. Negative effects on the leaves of submerged macrophyte and associated biofilms growth at high nitrate induced-stress. Aquat. Toxicol. 2020, 226, 105559. [Google Scholar] [CrossRef]
- Leland, H.V. Distribution of phytobenthos in the Yakima River basin, Washington, in relation to geology, land use and other environmental factors. Can. J. Fish. Aquat. Sci. 1995, 52, 1108–1129. [Google Scholar] [CrossRef]
- Walker, C.E.; Pan, Y. Using Diatom Assemblages to Assess Urban Stream Conditions. Hydrobiologia 2006, 561, 179–189. [Google Scholar] [CrossRef]
- Shen, X.; Li, R.; Chai, M.; Cheng, S.; Niu, Z.; Qiu, G.Y. Interactive effects of single, binary and trinary trace metals (lead, zinc and copper) on the physiological responses of Kandelia obovata seedlings. Environ. Geochem. Health 2019, 41, 135–148. [Google Scholar] [CrossRef]
- Zhang, J.W.; Huang, D.Y.; Deng, H.; Zhang, J.B. Responses of submerged plant Vallisneria natans growth and leaf biofilms to water contaminated with microplastics. Sci. Total Environ. 2022, 818, 151750. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Jiang, W.; Chen, J.; Chen, Y.; Zhang, X.; Wang, G. Microplastics with cadmium inhibit the growth of Vallisneria natans (Lour.) Hara rather than reduce cadmium toxicity. Chemosphere 2021, 266, 128979. [Google Scholar] [CrossRef]
- Li, Q.; Gu, P.; Zhang, H.; Luo, X.; Zhang, J.B.; Zheng, Z. Response of submerged macrophytes and leaf biofilms to the decline phase of Microcystis aeruginosa: Antioxidant response, ultrastructure, microbial properties, and potential mechanism. Sci. Total Environ. 2020, 699, 134325. [Google Scholar] [CrossRef]
- Hai-ting, J.; Dong, X.; Heng-jie, Z.; Shu-qing, A. Effects of leaf extracts from two kinds of submerged macrophytes on the growth and community composition of epiphytic algae living on native macrophyte species. Chin. J. Ecol. 2013, 32, 2298–2306. [Google Scholar]
- Dunck, B.; Nogueira, I.; Felisberto, S. Distribution of periphytic algae in wetlands (Palm swamps, Cerrado), Brazil. Braz. J. Biol. 2013, 73, 331–346. [Google Scholar] [CrossRef] [Green Version]







| Parameters | Maojiabu Lake | Xiaonanhu Lake | ||||
|---|---|---|---|---|---|---|
| Min–Max Value | Mean ± SD | Min-Max Value | Mean ± SD | F Values between Lakes | p Values between Lakes | |
| TP (mg/L) | 0.02–0.06 | 0.03 ± 0.02 | 0.01–0.09 | 0.05 ± 0.03 | −3.395 | 0.003 |
| TN (mg/L) | 1.48–4.34 | 2.27 ± 0.95 | 1.28–2.95 | 2.09 ± 0.57 | 0.672 | 0.507 |
| NO3−-N (mg/L) | 1.01–3.56 | 2.01 ± 0.71 | 1.18–2.55 | 1.79 ± 0.40 | 1.152 | 0.260 |
| NO2−-N (mg/L) | 0.03–0.07 | 0.04 ± 0.01 | 0.02–0.09 | 0.05 ± 0.02 | −1.249 | 0.223 |
| NH4+-N (mg/L) | 0.15–0.53 | 0.31 ± 0.12 | 0.09–0.78 | 0.29 ± 0.17 | −0.826 | 0.416 |
| Chl.a (μg/L) | 0.18–2.61 | 1.36 ± 0.77 | 0.18–2.95 | 1.01 ± 0.79 | −0.377 | 0.709 |
| DO (mg/L) | 5.74–6.80 | 6.40 ± 1.04 | 5.42–6.13 | 5.91 ± 2.19 | 0.417 | 0.680 |
| pH | 6.94–9.25 | 8.23 ± 0.72 | 7.47–8.22 | 7.89 ± 0.22 | 0.399 | 0.693 |
| EC (μs/cm) | 152.10–265.00 | 201.29 ± 27.66 | 146.10–221.00 | 184.57± 20.67 | 0.415 | 0.681 |
| ORP | 56.80–218.60 | 138.77 ± 49.23 | 78.40–228.90 | 156.86 ± 47.96 | 0.869 | 0.393 |
| TDS (mg/L) | 94.20–132.00 | 115.33 ± 11.00 | 84.00–134.20 | 106.83 ± 14.89 | 0.013 | 0.99 |
| WT (℃) | 7.80–26.60 | 17.46 ± 6.43 | 8.30–25.60 | 17.16 ± 5.81 | −0.004 | 0.997 |
| Water depth (m) | 0.70–0.90 | 0.78 ± 0.06 | 0.70–0.90 | 0.80 ± 0.06 | 0.322 | 0.750 |
| Turbidity (NTU) | 0.80–7.20 | 2.91 ± 1.79 | 0.70–11.40 | 3.08 2.89 | −0.100 | 0.921 |
| Substrates | Time | Substrates × Time | ||||
|---|---|---|---|---|---|---|
| Parameters | F Value | p Value | F Value | p Value | F Value | p Value |
| Species richness | 2.089 | 0.047 | 1.277 | 0.310 | 0.795 | 0.579 |
| Cell density | −4.943 | 0.000 | 2.600 | 0.048 | 7.226 | 0.000 |
| Chl.a | −2.514 | 0.025 | 3.955 | 0.008 | 4.898 | 0.001 |
| Shannon–Wiener diversity index | 1.387 | 0.181 | 0.885 | 0.523 | 25.273 | 0.000 |
| Jaccard similarity coefficient | 2.544 | 0.021 | 0.403 | 0.869 | 0.521 | 0.783 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Huang, S.; Yi, K.; Zhang, L.; Ge, F.; Lin, Q.; Zhang, Y.; Wu, Z.; Liu, B. Dynamic Characteristics of Periphytic Algae Communities on Different Substrates and the Host Response in Subtropical-Urban-Landscape Lakes. Water 2023, 15, 639. https://doi.org/10.3390/w15040639
Peng X, Huang S, Yi K, Zhang L, Ge F, Lin Q, Zhang Y, Wu Z, Liu B. Dynamic Characteristics of Periphytic Algae Communities on Different Substrates and the Host Response in Subtropical-Urban-Landscape Lakes. Water. 2023; 15(4):639. https://doi.org/10.3390/w15040639
Chicago/Turabian StylePeng, Xue, Suzhen Huang, Kelang Yi, Lu Zhang, Fangjie Ge, Qingwei Lin, Yi Zhang, Zhenbin Wu, and Biyun Liu. 2023. "Dynamic Characteristics of Periphytic Algae Communities on Different Substrates and the Host Response in Subtropical-Urban-Landscape Lakes" Water 15, no. 4: 639. https://doi.org/10.3390/w15040639