Climate Change and Reproductive Biocomplexity in Fishes: Innovative Management Approaches towards Sustainability of Fisheries and Aquaculture
Abstract
:1. Introduction
2. Climatic and Aquatic System Changes
3. Climatic Change Effects on Fish Reproduction
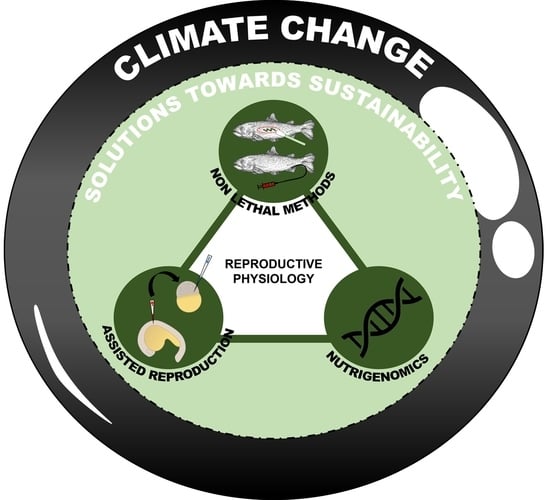
4. Climate Change and Solutions towards Sustainability
4.1. Non-Invasive and Non-Lethal Antioxidative Defense Monitoring and Sustainability
4.2. Assisted Reproduction
4.3. Nutrigenomics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pörtner, H.-O.; Roberts, D.C.; Adams, H.; Adler, C.; Aldunce, P.; Ali, E.; Begum, R.A.; Betts, R.; Kerr, R.B.; Biesbroek, R. Climate Change 2022: Impacts, Adaptation and Vulnerability; IPCC Sixth Assessment Report; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; Available online: https://www.ipcc.ch/report/ar6/wg2/downloads/report/IPCC_AR6_WGII_SummaryVolume.pdf (accessed on 1 January 2023).
- Froehlich, H.E.; Koehn, J.Z.; Holsman, K.K.; Halpern, B.S. Emerging Trends in Science and News of Climate Change Threats to and Adaptation of Aquaculture. Aquaculture 2022, 549, 737812. [Google Scholar] [CrossRef]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological Responses to Recent Climate Change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Barange, M.; Perry, R.I. Physical and Ecological Impacts of Climate Change Relevant to Marine and Inland Capture Fisheries and Aquaculture. In Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; Cochrane, K., de Young, C., Soto, D., Bahri, T., Eds.; FAO: Rome, Italy, 2009; pp. 7–106. Available online: https://www.cakex.org/sites/default/files/documents/i0994e02a.pdf (accessed on 1 January 2023).
- De Silva, S.S.; Soto, D. Climate Change and Aquaculture: Potential Impacts, Adaptation and Mitigation. In Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2009; Volume 530, pp. 151–212. [Google Scholar]
- Asch, R.G.; Stock, C.A.; Sarmiento, J.L. Climate Change Impacts on Mismatches between Phytoplankton Blooms and Fish Spawning Phenology. Glob. Chang. Biol. 2019, 25, 2544–2559. [Google Scholar] [CrossRef] [PubMed]
- Parisi, C.; Guerriero, G. Antioxidative Defense and Fertility Rate in the Assessment of Reprotoxicity Risk Posed by Global Warming. Antioxidants 2019, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chatterjee, S.; Shiva Prasad, G.; Pal, P. Effect of Climate Change on Aquatic Ecosystem and Production of Fisheries. In Inland Waters—Dynamics and Ecology; Devlin, A., Pan, J., Manjur Shah, M., Eds.; IntechOpen: Rijeka, Croatia, 2021; ISBN 978-1-83968-294-0. [Google Scholar]
- Muhala, V.; Chicombo, T.F.; Macate, I.E.; Guimarães-Costa, A.; Gundana, H.; Malichocho, C.; Hasimuna, O.J.; Remédio, A.; Maulu, S.; Cuamba, L.; et al. Climate Change in Fisheries and Aquaculture: Analysis of the Impact Caused by Idai and Kenneth Cyclones in Mozambique. Front. Sustain. Food Syst. 2021, 5, 714187. [Google Scholar] [CrossRef]
- Cussac, V.E.; Fernández, D.A.; Gómez, S.E.; López, H.L. Fishes of Southern South America: A Story Driven by Temperature. Fish Physiol. Biochem. 2009, 35, 29–42. [Google Scholar] [CrossRef]
- Brander, K. Effects of Environmental Variability on Growth and Recruitment in Cod (Gadus morhua) Using a Comparative Approach. Oceanol. Acta 2000, 23, 485–496. [Google Scholar] [CrossRef]
- Froese, R.; Papaioannou, E.; Scotti, M. Climate Change or Mismanagement? Environ. Biol. Fish. 2022, 105, 1363–1380. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022; ISBN 978-92-5-136364-5. [Google Scholar]
- Volpedo, A. Environmental Changes on Freshwater Fish Communities in South America in the Last Five Decades: A Case Study in Northeast Argentina. Sust. Agric. Food Environ. Res. 2016, 4, 47–61. [Google Scholar] [CrossRef]
- Oyinlola, M.A.; Reygondeau, G.; Wabnitz, C.C.C.; Frölicher, T.L.; Lam, V.W.Y.; Cheung, W.W.L. Projecting Global Mariculture Production and Adaptation Pathways under Climate Change. Glob. Chang. Biol. 2022, 28, 1315–1331. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; Porter, M.J.R. Cold and Dark or Warm and Light: Variations on the Theme of Environmental Control of Reproduction. Fish Physiol. Biochem. 2003, 28, 385–389. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Buckley, L.B. Quantifying Thermal Extremes and Biological Variation to Predict Evolutionary Responses to Changing Climate. Phil. Trans. R. Soc. B 2017, 372, 20160147. [Google Scholar] [CrossRef] [PubMed]
- Shama, L.N.S.; Wegner, K.M. Grandparental Effects in Marine Sticklebacks: Transgenerational Plasticity across Multiple Generations. J. Evol. Biol. 2014, 27, 2297–2307. [Google Scholar] [CrossRef] [PubMed]
- Audzijonyte, A.; Fulton, E.; Haddon, M.; Helidoniotis, F.; Hobday, A.J.; Kuparinen, A.; Morrongiello, J.; Smith, A.D.; Upston, J.; Waples, R.S. Trends and Management Implications of Human-Influenced Life-History Changes in Marine Ectotherms. Fish Fish. 2016, 17, 1005–1028. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate Change Effects on Biodiversity, Ecosystems, Ecosystem Services, and Natural Resource Management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Plagányi, É. Climate Change Impacts on Fisheries. Science 2019, 363, 930–931. [Google Scholar] [CrossRef]
- Gentilucci, M.; Parisi, C.; Coppola, M.R.; Majdoubi, F.-Z.; Madonna, A.; Guerriero, G. Influence of Mediterranean Sea Temperature Increase on Gaeta Gulf (Tyrrhenian Sea) Biodiversity. Proc. Zool. Soc. 2021, 74, 91–103. [Google Scholar] [CrossRef]
- Muringai, R.T.; Mafongoya, P.L.; Lottering, R. Climate Change and Variability Impacts on Sub-Saharan African Fisheries: A Review. Rev. Fish. Sci. Aquac. 2021, 29, 706–720. [Google Scholar] [CrossRef]
- Hiddink, J.G.; Ter Hofstede, R. Climate Induced Increases in Species Richness of Marine Fishes: Climate change and fish species richness. Glob. Chang. Biol. 2008, 14, 453–460. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Rabaoui, L.; Basali, A.U.; Lopez, M.; Lindo, R.; Krishnakumar, P.K.; Qurban, M.A.; Prihartato, P.K.; Cortes, D.L.; Qasem, A.; et al. Long-Term Ecological Changes in Fishes and Macro-Invertebrates in the World’s Warmest Coral Reefs. Sci. Total Environ. 2021, 750, 142254. [Google Scholar] [CrossRef]
- Colautti, D.; Baigún, C.; Llompart, F.; Maiztegui, T.; Garcia de Souza, J.; Solimano, P.; Balboni, L.; Berasain, G. Fish Assemblage of a Pampean Shallow Lake, a Story of Instability. Hydrobiologia 2015, 752, 175–186. [Google Scholar] [CrossRef]
- Santos, D.; Luzio, A.; Coimbra, A.M. Zebrafish Sex Differentiation and Gonad Development: A Review on the Impact of Environmental Factors. Aquat. Toxicol. 2017, 191, 141–163. [Google Scholar] [CrossRef]
- Alix, M.; Kjesbu, O.S.; Anderson, K.C. From Gametogenesis to Spawning: How Climate-driven Warming Affects Teleost Reproductive Biology. J. Fish. Biol. 2020, 97, 607–632. [Google Scholar] [CrossRef]
- Feugere, L.; Scott, V.F.; Rodriguez-Barucg, Q.; Beltran-Alvarez, P.; Wollenberg Valero, K.C. Thermal Stress Induces a Positive Phenotypic and Molecular Feedback Loop in Zebrafish Embryos. J. Therm. Biol. 2021, 102, 103114. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, M.; Lu, Z.-Y.; Liu, Y.; Li, B.; Gao, Z.-X.; Shen, Z.-G. High-Temperature Stress Will Put the Thermo-Sensitive Teleost Yellow Catfish (Tachysurus Fulvidraco) in Danger through Reducing Reproductivity. Ecotoxicol. Environ. Saf. 2022, 239, 113638. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, N.W.; Munday, P.L. Effects of Climate Change on Fish Reproduction and Early Life History Stages. Mar. Freshw. Res. 2011, 62, 1015–1026. [Google Scholar] [CrossRef]
- Lindmark, M.; Audzijonyte, A.; Blanchard, J.L.; Gårdmark, A. Temperature Impacts on Fish Physiology and Resource Abundance Lead to Faster Growth but Smaller Fish Sizes and Yields under Warming. Glob. Chang. Biol. 2022, 28, 6239–6253. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G. Vertebrate Sex Steroid Receptors: Evolution, Ligands, and Neurodistribution. Ann. N. Y. Acad. Sci. 2009, 1163, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Roselli, C.E.; Paolucci, M.; Botte, V.; Ciarcia, G. Estrogen Receptors and Aromatase Activity in the Hypothalamus of the Female Frog, Rana Esculenta. Fluctuations throughout the Reproductive Cycle. Brain Res. 2000, 880, 92–101. [Google Scholar] [CrossRef]
- Guerriero, G.; Roselli, C.E.; Ciarcia, G. The Amphibian (Rana esculenta) Brain Progesterone Receptor: Relationship to Plasma Steroids and Vitellogenic Cycle during the Gonadal Recovery Phase. Ann. N. Y. Acad. Sci. 2009, 1163, 407–409. [Google Scholar] [CrossRef]
- Miranda, L.A.; Chalde, T.; Elisio, M.; Strüssmann, C.A. Effects of Global Warming on Fish Reproductive Endocrine Axis, with Special Emphasis in Pejerrey Odontesthes Bonariensis. Gen. Comp. Endocrinol. 2013, 192, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cueto, J.A.; Zmora, N.; Paullada-Salmerón, J.A.; Marvel, M.; Mañanos, E.; Zohar, Y. The Gonadotropin-Releasing Hormones: Lessons from Fish. Gen. Comp. Endocrinol. 2020, 291, 113422. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Trocchia, S.; Abdel-Gawad, F.K.; Ciarcia, G. Roles of Reactive Oxygen Species in the Spermatogenesis Regulation. Front. Endocrinol. 2014, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Luis Val, A.; Wood, C.M. Global Change and Physiological Challenges for Fish of the Amazon Today and in the near Future. J. Exp. Biol. 2022, 225, jeb216440. [Google Scholar] [CrossRef] [PubMed]
- Lema, S.C.; Chow, M.I.; Dittman, A.H.; May, D.; Housh, M.J. Accustomed to the Heat: Temperature and Thyroid Hormone Influences on Oogenesis and Gonadal Steroidogenesis Pathways Vary among Populations of Amargosa Pupfish (Cyprinodon Nevadensis Amargosae). Comp. Biochem. Physiol. Part A 2022, 272, 111280. [Google Scholar] [CrossRef] [PubMed]
- Haworth, M.R.; Bestgen, K.R. Flow and Water Temperature Affect Reproduction and Recruitment of a Great Plains Cyprinid. Can. J. Fish. Aquat. Sci. 2017, 74, 853–863. [Google Scholar] [CrossRef]
- Morales-Marín, L.A.; Rokaya, P.; Sanyal, P.R.; Sereda, J.; Lindenschmidt, K.E. Changes in Streamflow and Water Temperature Affect Fish Habitat in the Athabasca River Basin in the Context of Climate Change. Ecol. Model. 2019, 407, 108718. [Google Scholar] [CrossRef]
- Fisher, M.C.; Helser, T.E.; Kang, S.; Gwak, W.; Canino, M.F.; Hauser, L. Genetic Structure and Dispersal in Peripheral Populations of a Marine Fish (Pacific Cod, Gadus macrocephalus) and Their Importance for Adaptation to Climate Change. Ecol. Evol. 2022, 12, e8474. [Google Scholar] [CrossRef]
- Fraser, G.S.; Bestgen, K.R.; Winkelman, D.L.; Thompson, K.G. Temperature—Not Flow—Predicts Native Fish Reproduction with Implications for Climate Change. Trans. Am. Fish. Soc. 2019, 148, 509–527. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Farrell, A.P. Physiology and Climate Change. Science 2008, 322, 690–692. [Google Scholar] [CrossRef]
- Frommel, A.Y.; Lye, S.L.R.; Brauner, C.J.; Hunt, B.P.V. Air Exposure Moderates Ocean Acidification Effects during Embryonic Development of Intertidally Spawning Fish. Sci. Rep. 2022, 12, 12270. [Google Scholar] [CrossRef] [PubMed]
- Porteus, C.S.; Hubbard, P.C.; Uren Webster, T.M.; van Aerle, R.; Canário, A.V.M.; Santos, E.M.; Wilson, R.W. Near-Future CO2 Levels Impair the Olfactory System of a Marine Fish. Nat. Clim. Chang. 2018, 8, 737–743. [Google Scholar] [CrossRef]
- Biswal, A.; Srivastava, P.P.; Krishna, G.; Paul, T.; Pal, P.; Gupta, S.; Varghese, T.; Jayant, M. An Integrated Biomarker Approach for Explaining the Potency of Exogenous Glucose on Transportation Induced Stress in Labeo Rohita Fingerlings. Sci. Rep. 2021, 11, 5713. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.; Downes, B.J.; Swearer, S.E. Habitat Selection as a Source of Inter-Specific Differences in Recruitment of Two Diadromous Fish Species. Freshw. Biol. 2008, 53, 2145–2157. [Google Scholar] [CrossRef]
- Lehtonen, T.K.; Wong, B.B.M.; Kvarnemo, C. Effects of Salinity on Nest-Building Behaviour in a Marine Fish. BMC Ecol. 2016, 16, 7. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Sandker, J.F.; van de Pol, I.L.E.; Urbina, M.A.; Wilson, R.W.; McKenzie, D.J.; Leiva, F.P. Body Mass and Cell Size Shape the Tolerance of Fishes to Low Oxygen in a Temperature-dependent Manner. Glob. Chang. Biol. 2022, 28, 5695–5707. [Google Scholar] [CrossRef]
- Cruz Vieira, A.B.; Weber, A.A.; Ribeiro, Y.M.; Luz, R.K.; Bazzoli, N.; Rizzo, E. Influence of Salinity on Spermatogenesis in Adult Nile Tilapia (Oreochromis Niloticus) Testis. Theriogenology 2019, 131, 1–8. [Google Scholar] [CrossRef]
- Craig, L.S.; Olden, J.D.; Arthington, A.H.; Entrekin, S.; Hawkins, C.P.; Kelly, J.J.; Kennedy, T.A.; Maitland, B.M.; Rosi, E.J.; Roy, A.H.; et al. Meeting the Challenge of Interacting Threats in Freshwater Ecosystems: A Call to Scientists and Managers. Elementa 2017, 5, 72. [Google Scholar] [CrossRef]
- Iglesias, M.C.-A. A Review of Recent Advances and Future Challenges in Freshwater Salinization. Limnetica 2020, 39, 185–211. [Google Scholar]
- Valdivieso, A.; Wilson, C.A.; Amores, A.; da Silva Rodrigues, M.; Nóbrega, R.H.; Ribas, L.; Postlethwait, J.H.; Piferrer, F. Environmentally-Induced Sex Reversal in Fish with Chromosomal vs. Polygenic Sex Determination. Environ. Res. 2022, 213, 113549. [Google Scholar] [CrossRef]
- Gentilucci, M.; Moustafa, A.A.; Abdel-Gawad, F.K.; Mansour, S.R.; Coppola, M.R.; Caserta, L.; Inglese, S.; Pambianchi, G.; Guerriero, G. Advances in Egyptian Mediterranean Coast Climate Change Monitoring. Water 2021, 13, 1870. [Google Scholar] [CrossRef]
- Silvano, R.A.M.; Begossi, A. Fishermen’s Local Ecological Knowledge on Southeastern Brazilian Coastal Fishes: Contributions to Research, Conservation, and Management. Neotrop. Ichthyol. 2012, 10, 133–147. [Google Scholar] [CrossRef]
- Hare, J.A.; Morrison, W.E.; Nelson, M.W.; Stachura, M.M.; Teeters, E.J.; Griffis, R.B.; Alexander, M.A.; Scott, J.D.; Alade, L.; Bell, R.J.; et al. A Vulnerability Assessment of Fish and Invertebrates to Climate Change on the Northeast U.S. Continental Shelf. PLoS ONE 2016, 11, e0146756. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.D.; Hollowed, A.B.; Sigler, M.F.; Hermann, A.J.; Nelson, M.W. Trait-based Climate Vulnerability Assessments in Data-rich Systems: An Application to Eastern Bering Sea Fish and Invertebrate Stocks. Glob. Chang. Biol. 2019, 25, 3954–3971. [Google Scholar] [CrossRef] [PubMed]
- Karr, J.R.; Larson, E.R.; Chu, E.W. Ecological Integrity Is Both Real and Valuable. Conserv. Sci. Pract. 2022, 4, e583. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, Q.; Cao, J.; Jin, Y.; Zhao, N.; Xu, W.; Liu, H.; Tang, H.; Qiao, Y.; Chen, X. Using a Hierarchical Model Framework to Investigate the Relationships between Fish Spawning and Abiotic Factors for Environmental Flow Management. Sci. Total Environ. 2021, 787, 147618. [Google Scholar] [CrossRef]
- Cornejo-Donoso, J.; Einarsson, B.; Birnir, B.; Gaines, S.D. Effects of Fish Movement Assumptions on the Design of a Marine Protected Area to Protect an Overfished Stock. PLoS ONE 2017, 12, e0186309. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.D.; Cisneros-Montemayor, A.; Hanich, Q.; Johnson, J.E.; Lehodey, P.; Moore, B.R.; Pratchett, M.S.; Reygondeau, G.; Senina, I.; Virdin, J.; et al. Adaptations to Maintain the Contributions of Small-Scale Fisheries to Food Security in the Pacific Islands. Mar. Policy 2018, 88, 303–314. [Google Scholar] [CrossRef]
- Raworth, K. Doughnut Economics: Seven Ways to Think Like a 21st-Century Economist; Chelsea Green Publishing: White River Junction, VT, USA, 2017. [Google Scholar]
- Loring, P.A.; Fazzino, D.V.; Agapito, M.; Chuenpagdee, R.; Gannon, G.; Isaacs, M. Fish and Food Security in Small-Scale Fisheries. In Transdisciplinarity for Small-Scale Fisheries Governance; Chuenpagdee, R., Jentoft, S., Eds.; MARE Publication Series; Springer International Publishing: Cham, Switzerland, 2019; Volume 21, pp. 55–73. ISBN 978-3-319-94937-6. [Google Scholar]
- Meester, L.D.; Stoks, R.; Brans, K.I. Genetic Adaptation as a Biological Buffer against Climate Change: Potential and Limitations. Integr. Zool. 2018, 13, 372–391. [Google Scholar] [CrossRef]
- Bell, J.D.; Ganachaud, A.; Gehrke, P.C.; Griffiths, S.P.; Hobday, A.J.; Hoegh-Guldberg, O.; Johnson, J.E.; Le Borgne, R.; Lehodey, P.; Lough, J.M.; et al. Mixed Responses of Tropical Pacific Fisheries and Aquaculture to Climate Change. Nat. Clim. Chang. 2013, 3, 591–599. [Google Scholar] [CrossRef]
- Radchuk, V.; Reed, T.; Teplitsky, C.; van de Pol, M.; Charmantier, A.; Hassall, C.; Adamík, P.; Adriaensen, F.; Ahola, M.P.; Arcese, P.; et al. Adaptive Responses of Animals to Climate Change Are Most Likely Insufficient. Nat. Commun. 2019, 10, 3109. [Google Scholar] [CrossRef] [PubMed]
- Denechaud, C.; Smoliński, S.; Geffen, A.J.; Godiksen, J.A.; Campana, S.E. A Century of Fish Growth in Relation to Climate Change, Population Dynamics and Exploitation. Glob. Chang. Biol. 2020, 26, 5661–5678. [Google Scholar] [CrossRef] [PubMed]
- Lavin, C.P.; Jones, G.P.; Williamson, D.H.; Harrison, H.B. Minimum Size Limits and the Reproductive Value of Numerous, Young, Mature Female Fish. Proc. R. Soc. B 2021, 288, 20202714. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.A.; Crichigno, S.A.; Cussac, V.E. Climate Change Impacts on Freshwater Fishes: A Patagonian Perspective. Hydrobiologia 2018, 816, 21–38. [Google Scholar] [CrossRef]
- Ouizgane, A.; Farid, S.; Majdoubi, F.Z.; Droussi, M.; Guerriero, G.; Hasnaoui, M. Assessment of Climate Change Effects on Predation Activity and Growth of Largemouth Bass, Micropterus Salmoides (Lacepède, 1802) by Water Temperature Variations. Emir. J. Food Agric. 2018, 30, 515–521. [Google Scholar] [CrossRef]
- Gibson, D.; Riecke, T.V.; Catlin, D.H.; Hunt, K.L.; Weithman, C.E.; Koons, D.N.; Karpanty, S.M.; Fraser, J.D. Climate Change and Commercial Fishing Practices Codetermine Survival of a Long-lived Seabird. Glob. Chang. Biol. 2022, 29, 324–340. [Google Scholar] [CrossRef]
- Knowles, J.; Vysloužil, J.; Policar, T.; Milla, S.; Holická, M.; Podhorec, P. Spawning Performance and Sex Steroid Levels in Female Pikeperch Sander Lucioperca Treated with Poly(Lactic-Co-Glycolic Acid) Microparticles. Animals 2022, 12, 208. [Google Scholar] [CrossRef]
- Shima, J.S.; Osenberg, C.W.; Alonzo, S.H.; Noonburg, E.G.; Mitterwallner, P.; Swearer, S.E. Reproductive Phenology across the Lunar Cycle: Parental Decisions, Offspring Responses, and Consequences for Reef Fish. Ecology 2020, 101, e03086. [Google Scholar] [CrossRef]
- Majdoubi, F.-Z.; Ouizgane, A.; Farid, S.; Mossetti, S.L.; Droussi, M.; Guerriero, G.; Hasnaoui, M. Fry Survival Rate as a Predictive Marker of Optimal Production of Silver Carp (Hypophthalmichthys molitrix, Valenciennes 1844): A Biostatistical Study in Deroua Fish Farm, Morocco. Proc. Zool. Soc. 2022, 75, 152–160. [Google Scholar] [CrossRef]
- Wootton, H.F.; Audzijonyte, A.; Morrongiello, J. Multigenerational Exposure to Warming and Fishing Causes Recruitment Collapse, but Size Diversity and Periodic Cooling Can Aid Recovery. Proc. Natl. Acad. Sci. USA 2021, 118, e2100300118. [Google Scholar] [CrossRef]
- Wilson, M.G.; Lavis, J.N.; Travers, R.; Rourke, S.B. Community-Based Knowledge Transfer and Exchange: Helping Community-Based Organizations Link Research to Action. Implement. Sci. 2010, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Brosset, P.; Smith, A.D.; Plourde, S.; Castonguay, M.; Lehoux, C.; Van Beveren, E. A Fine-Scale Multi-Step Approach to Understand Fish Recruitment Variability. Sci. Rep. 2020, 10, 16064. [Google Scholar] [CrossRef] [PubMed]
- Oyebola, O.O.; Efitre, J.; Musinguzi, L.; Falaye, A.E. Potential Adaptation Strategies for Climate Change Impact among Flood-Prone Fish Farmers in Climate Hotspot Uganda. Environ. Dev. Sustain. 2021, 23, 12761–12790. [Google Scholar] [CrossRef]
- Mitra, A.; Mukhopadhyay, P.K.; Homechaudhuri, S. An Overview of Biology And Culture Potentials of Humped Featherback Chitala chitala (Hamilton, 1822)—A New Candidate for Aquaculture Diversification. Rev. Fish. Sci. Aquac. 2018, 26, 371–380. [Google Scholar] [CrossRef]
- D’Errico, G.; Vitiello, G.; De Tommaso, G.; Abdel-Gawad, F.K.; Brundo, M.V.; Ferrante, M.; De Maio, A.; Trocchia, S.; Bianchi, A.R.; Ciarcia, G.; et al. Electron Spin Resonance (ESR) for the Study of Reactive Oxygen Species (ROS) on the Isolated Frog Skin (Pelophylax Bergeri): A Non-Invasive Method for Environmental Monitoring. Environ. Res. 2018, 165, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; D’Errico, G. Effect of Oxidative Stress on Reproduction and Development. Antioxidants 2022, 11, 312. [Google Scholar] [CrossRef]
- Lacerda, S.M.S.N.; Costa, G.M.J.; Campos-Junior, P.H.A.; Segatelli, T.M.; Yazawa, R.; Takeuchi, Y.; Morita, T.; Yoshizaki, G.; França, L.R. Germ Cell Transplantation as a Potential Biotechnological Approach to Fish Reproduction. Fish Physiol. Biochem. 2013, 39, 3–11. [Google Scholar] [CrossRef]
- De Siqueira-Silva, D.H.; Saito, T.; dos Santos-Silva, A.P.; da Silva Costa, R.; Psenicka, M.; Yasui, G.S. Biotechnology Applied to Fish Reproduction: Tools for Conservation. Fish Physiol. Biochem. 2018, 44, 1469–1485. [Google Scholar] [CrossRef]
- Martin, S.A.M.; Król, E. Nutrigenomics and Immune Function in Fish: New Insights from Omics Technologies. Dev. Comp. Immunol. 2017, 75, 86–98. [Google Scholar] [CrossRef]
- Hakim, M.M.; Ganai, N.A.; Ahmad, S.M.; Asmi, O.; Akram, T.; Hussain, S.; Gora, A.H. Nutrigenomics: Omics Approach in Aquaculture Research to Mitigate the Deficits in Conventional Nutritional Practices. J. Entomol. Zool. Stud. 2018, 6, 582–587. [Google Scholar]
- Madeira, D.; Vinagre, C.; Diniz, M.S. Are Fish in Hot Water? Effects of Warming on Oxidative Stress Metabolism in the Commercial Species Sparus Aurata. Ecol. Indic. 2016, 63, 324–331. [Google Scholar] [CrossRef]
- Aliko, V.; Qirjo, M.; Sula, E.; Morina, V.; Faggio, C. Antioxidant Defense System, Immune Response and Erythron Profile Modulation in Gold Fish, Carassius Auratus, after Acute Manganese Treatment. Fish Shellfish Immunol. 2018, 76, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, F.K.; Khalil, W.K.B.; Bassem, S.M.; Kumar, V.; Parisi, C.; Inglese, S.; Temraz, T.A.; Nassar, H.F.; Guerriero, G. The Duckweed, Lemna Minor Modulates Heavy Metal-Induced Oxidative Stress in the Nile Tilapia, Oreochromis Niloticus. Water 2020, 12, 2983. [Google Scholar] [CrossRef]
- Parisi, C.; De Marco, G.; Labar, S.; Hasnaoui, M.; Grieco, G.; Caserta, L.; Inglese, S.; Vangone, R.; Madonna, A.; Alwany, M.; et al. Biodiversity Studies for Sustainable Lagoon: Thermophilic and Tropical Fish Species vs. Endemic Commercial Species at Mellah Lagoon (Mediterranean, Algeria). Water 2022, 14, 635. [Google Scholar] [CrossRef]
- Council of the European Union. Directive 86/609/EEC of 24 November 1986 on the Approximation of Laws, Regulations and Administrative Provisions of the Member States Regarding the Protection of Animals Used for Experimental and Other Scientific Purposes. Off. J. Eur. Communities 1986, 358, 1–29. Available online: http://data.europa.eu/eli/dir/1986/609/oj (accessed on 1 January 2023).
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Guerriero, G.; Ferrara, C.; Ciarcia, G. From Rainbow Trout Skin Biopsy to Potential Adverse Health Effects Assessment. Free Radic. Biol. Med. 2012, 53, S135. [Google Scholar] [CrossRef]
- Guezgouz, N.; Parisi, C.; Boubsil, S.; Grieco, G.; Hana, S.A.; Guerriero, G. Heavy Metals Assessment in the Medjerda River Basin (Northeastern Algeria): A Preliminary Water Analysis and Toad Skin Biopsy. Proc. Zool. Soc. 2021, 74, 104–113. [Google Scholar] [CrossRef]
- Reverter, M.; Tapissier-Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Biological and Ecological Roles of External Fish Mucus: A Review. Fishes 2018, 3, 41. [Google Scholar] [CrossRef]
- Ross, N.W.; Firth, K.J.; Wang, A.; Burka, J.F.; Johnson, S.C. Changes in Hydrolytic Enzyme Activities of Naive Atlantic Salmon Salmo Salar Skin Mucus Due to Infection with the Salmon Louse Lepeophtheirus Salmonis and Cortisol Implantation. Dis. Aquat. Org. 2000, 41, 43–51. [Google Scholar] [CrossRef]
- Dzul-Caamal, R.; Olivares-Rubio, H.F.; López-Tapia, P.; Vega-López, A. Pro-Oxidant and Antioxidant Response Elicited by CH2Cl2, CHCl3 and BrCHCl2 in Goodea Gracilis Using Non-Invasive Methods. Comp. Biochem. Physiol. Part A 2013, 165, 515–527. [Google Scholar] [CrossRef]
- Gupta, G.; Srivastava, P.P.; Gangwar, M.; Varghese, T.; Chanu, T.I.; Gupta, S.; Ande, M.P.; Krishna, G.; Jana, P. Extra-Fortification of Zinc Upsets Vitellogenin Gene Expression and Antioxidant Status in Female of Clarias Magur Brooders. Biol. Trace Elem. Res. 2022, 200, 1861–1871. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; MacKinnon, S.L.; Ross, N.W. A Comparative Study on Innate Immune Parameters in the Epidermal Mucus of Various Fish Species. Comp. Biochem. Physiol. Part B 2007, 148, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Dzul-Caamal, R.; Salazar-Coria, L.; Olivares-Rubio, H.F.; Rocha-Gómez, M.A.; Girón-Pérez, M.I.; Vega-López, A. Oxidative Stress Response in the Skin Mucus Layer of Goodea Gracilis (Hubbs and Turner, 1939) Exposed to Crude Oil: A Non-Invasive Approach. Comp. Biochem. Physiol. Part A 2016, 200, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Dzul-Caamal, R.; Olivares-Rubio, H.F.; Salazar-Coria, L.; Rocha-Gómez, M.A.; Vega-López, A. Multivariate Analysis of Biochemical Responses Using Non-Invasive Methods to Evaluate the Health Status of the Endangered Blackfin Goodeid (Girardinichthys Viviparus). Ecol. Indic. 2016, 60, 1118–1129. [Google Scholar] [CrossRef]
- Soivio, A.; Nynolm, K.; Westman, K. A Technique for Repeated Sampling of the Blood of Individual Resting Fish. J. Exp. Biol. 1975, 63, 207–217. [Google Scholar] [CrossRef]
- Caldwell, S.; Rummer, J.L.; Brauner, C.J. Blood Sampling Techniques and Storage Duration: Effects on the Presence and Magnitude of the Red Blood Cell β-Adrenergic Response in Rainbow Trout (Oncorhynchus Mykiss). Comp. Biochem. Physiol. Part A 2006, 144, 188–195. [Google Scholar] [CrossRef]
- Pollard, S.; Anderson, J.; Bah, F.; Mateus, M.; Sidhu, M.; Simmons, D. Non-Lethal Blood Sampling of Fish in the Lab and Field With Methods for Dried Blood Plasma Spot Omic Analyses. Front. Genet. 2022, 13, 795348. [Google Scholar] [CrossRef]
- Goldstein, L.; Forster, R.P.; Fanelli, G.M. Gill Blood Flow and Ammonia Excretion in the Marine Teleost, Myoxocephalus Scorpius. Comp. Biochem. Physiol. 1964, 12, 489–499. [Google Scholar] [CrossRef]
- Kaleeswaran, B.; Ilavenil, S.; Karthik, D.; Ravikumar, S. A New Method of Approaching the Heart for Rapid Bleeding in Fish. Sijbs 2016, 2, 337. [Google Scholar] [CrossRef]
- Guerriero, G.; Di Finizio, A.; Ciarcia, G. Stress-Induced Changes of Plasma Antioxidants in Aquacultured Sea Bass, Dicentrarchus Labrax. Comp. Biochem. Physiol. Part A 2002, 132, 205–211. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Jain-Schlaepfer, S.; Zolderdo, A.J.; Algera, D.A.; Gilmour, K.M.; Gallagher, A.J.; Cooke, S.J. Are 3 Minutes Good Enough for Obtaining Baseline Physiological Samples from Teleost Fish? Can. J. Zool. 2018, 96, 774–786. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Raby, G.D.; Teffer, A.K.; Jeffries, K.M.; Danylchuk, A.J.; Eliason, E.J.; Hasler, C.T.; Clark, T.D.; Cooke, S.J. Best Practices for Non-lethal Blood Sampling of Fish via the Caudal Vasculature. J. Fish Biol. 2020, 97, 4–15. [Google Scholar] [CrossRef]
- Guerriero, G.; Ferro, R.; Ciarcia, G. Correlations between Plasma Levels of Sex Steroids and Spermatogenesis during the Sexual Cycle of the Chub, Leuciscus cephalus L. (Pisces: Cyprinidae). Zool. Stud. 2005, 44, 228–233. [Google Scholar]
- Shao, X.; Liu, W.; Xu, W.; Lu, K.; Xia, W.; Jiang, Y. Effects of Dietary Copper Sources and Levels on Performance, Copper Status, Plasma Antioxidant Activities and Relative Copper Bioavailability in Carassius Auratus Gibelio. Aquaculture 2010, 308, 60–65. [Google Scholar] [CrossRef]
- Flett, P.A.; Kraak, G.V.D.; Munkittrick, K.R.; Leatherland, J.F. Overripening as the Cause of Low Survival to Hatch in Lake Erie Coho Salmon (Oncorhynchus kisutch) Embryos. Can. J. Zool. 1996, 74, 851–857. [Google Scholar] [CrossRef]
- Guerriero, G.; Paolucci, M.; Bianco, P.G.; Botte, V.; Ciarcia, G. The Reproductive Cycle of the Endangered Cyprinid Alburnus albidus: Morphological Changes of the Gonads and Plasma Sex Steroid Fluctuations. Ital. J. Zool. 1998, 65, 223–226. [Google Scholar] [CrossRef]
- Pankhurst, N.W.; King, H.R. Temperature and Salmonid Reproduction: Implications for Aquaculture. J. Fish Biol. 2010, 76, 69–85. [Google Scholar] [CrossRef]
- Majdoubi, F.-Z.; Benhima, R.; Ouizgane, A.; Farid, S.; Droussi, M.; Guerriero, G.; Hasnaoui, M. Ova Fatty Acids Composition and Spawning Performances of Silver Carp, Hypophthalmichthys Molitrix (Morocco). Turk. J. Fish. Aquat. Sci. 2020, 20, 879–888. [Google Scholar] [CrossRef]
- Carrillo, M.; Zanuy, S.; Oyen, F.; Cerdá, J.; Navas, J.M.; Ramos, J. Some Criteria of the Quality of the Progeny as Indicators of Physiological Broodstock Fitness. In Recent Advances in Mediterranean Aquaculture Finfish Species Diversification; CIHEAM: Zaragoza, Spain, 2000; Available online: http://om.ciheam.org/om/pdf/c47/00600606.pdf (accessed on 1 January 2023).
- Venturelli, P.A.; Murphy, C.A.; Shuter, B.J.; Johnston, T.A.; van Coeverden de Groot, P.J.; Boag, P.T.; Casselman, J.M.; Montgomerie, R.; Wiegand, M.D.; Leggett, W.C. Maternal Influences on Population Dynamics: Evidence from an Exploited Freshwater Fish. Ecology 2010, 91, 2003–2012. [Google Scholar] [CrossRef]
- Macchi, G.J.; Leonarduzzi, E.; Diaz, M.V.; Renzi, M.; Rodrigues, K. Maternal Effects on Fecundity and Egg Quality of the Patagonian Stock of Argentine Hake (Merluccius hubbsi). Fish. Bull. 2013, 111, 325–336. [Google Scholar] [CrossRef]
- Margules, C.R.; Pressey, R.L. Systematic Conservation Planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Pukazhenthi, B.; Comizzoli, P.; Travis, A.J.; Wildt, D.E. Applications of Emerging Technologies to the Study and Conservation of Threatened and Endangered Species. Reprod. Fertil. Dev. 2006, 18, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Long, W.; Chen, J.; Hopkins, N. Production of Germ-Line Chimeras in Zebrafish by Cell Transplants from Genetically Pigmented to Albino Embryos. Proc. Natl. Acad. Sci. USA 1992, 89, 4519–4523. [Google Scholar] [CrossRef]
- Saito, T.; Goto-Kazeto, R.; Arai, K.; Yamaha, E. Xenogenesis in Teleost Fish Through Generation of Germ-Line Chimeras by Single Primordial Germ Cell Transplantation. Biol. Reprod. 2008, 78, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Tsutsumi, O.; Ikezuki, Y.; Takai, Y.; Taketani, Y. Positive Relationship between Androgen and the Endocrine Disruptor, Bisphenol A, in Normal Women and Women with Ovarian Dysfunction. Endocr. J 2004, 51, 165–169. [Google Scholar] [CrossRef]
- Okutsu, T.; Suzuki, K.; Takeuchi, Y.; Takeuchi, T.; Yoshizaki, G. Testicular Germ Cells Can Colonize Sexually Undifferentiated Embryonic Gonad and Produce Functional Eggs in Fish. Proc. Natl. Acad. Sci. USA 2006, 103, 2725–2729. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Santos, J.A.; Madrid, R.M. Copper Emission Factors from Intensive Shrimp Aquaculture. Mar. Pollut. Bull. 2006, 52, 1823–1826. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Fujinuma, K.; Iwasaki, Y.; Okutsu, T.; Shikina, S.; Yazawa, R.; Takeuchi, Y. Spermatogonial Transplantation in Fish: A Novel Method for the Preservation of Genetic Resources. Comp. Biochem. Physiol. Part D 2011, 6, 55–61. [Google Scholar] [CrossRef]
- Saito, T.; Fujimoto, T.; Maegawa, S.; Inoue, K.; Tanaka, M.; Arai, K.; Yamaha, E. Visualization of Primordial Germ Cells In Vivo Using GFP-Nos1 3’UTR MRNA. Int. J. Dev. Biol. 2006, 50, 691–699. [Google Scholar] [CrossRef]
- Majhi, S.K.; Kumar, S. Germ Cell Transplantation: A Potential Tool for Propagation of Endangered Fishes. Ann. Aquac. Res. 2017, 4, 4–7. [Google Scholar]
- Majhi, S.K.; Hattori, R.S.; Yokota, M.; Watanabe, S.; Strüssmann, C.A. Germ Cell Transplantation Using Sexually Competent Fish: An Approach for Rapid Propagation of Endangered and Valuable Germlines. PLoS ONE 2009, 4, e6132. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Xu, D.; Ino, Y.; Yoshino, T.; Hayashida, T.; Wang, J.; Yazawa, R.; Yoshizaki, G.; Takeuchi, Y. Hybrid Sterility in Fish Caused by Mitotic Arrest of Primordial Germ Cells. Genetics 2018, 209, 507–521. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Takeuchi, Y.; Ino, Y.; Wang, J.; Iwata, G.; Kabeya, N.; Yazawa, R.; Yoshizaki, G. Efficient Production of Donor-Derived Gametes from Triploid Recipients Following Intra-Peritoneal Germ Cell Transplantation into a Marine Teleost, Nibe Croaker (Nibea Mitsukurii). Aquaculture 2017, 478, 35–47. [Google Scholar] [CrossRef]
- Tani, R.; Kawamura, W.; Morita, T.; Klopp, C.; Milhes, M.; Guiguen, Y.; Yoshizaki, G.; Yazawa, R. Development of a Polymerase Chain Reaction (PCR)-Based Genetic Sex Identification Method in the Chub Mackerel Scomber Japonicus and Blue Mackerel S. australasicus. Fish. Sci. 2021, 87, 785–793. [Google Scholar] [CrossRef]
- Morita, T.; Kumakura, N.; Morishima, K.; Mitsuboshi, T.; Ishida, M.; Hara, T.; Kudo, S.; Miwa, M.; Ihara, S.; Higuchi, K.; et al. Production of Donor-Derived Offspring by Allogeneic Transplantation of Spermatogonia in the Yellowtail (Seriola quinqueradiata). Biol. Reprod. 2012, 86, 176. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, G.; Lee, S. Production of Live Fish Derived from Frozen Germ Cells via Germ Cell Transplantation. Stem Cell Res. 2018, 29, 103–110. [Google Scholar] [CrossRef]
- Goto, R.; Saito, T.; Takeda, T.; Fujimoto, T.; Takagi, M.; Arai, K.; Yamaha, E. Germ Cells Are Not the Primary Factor for Sexual Fate Determination in Goldfish. Dev. Biol. 2012, 370, 98–109. [Google Scholar] [CrossRef]
- Li, M.; Hong, N.; Xu, H.; Song, J.; Hong, Y. Germline Replacement by Blastula Cell Transplantation in the Fish Medaka. Sci. Rep. 2016, 6, 29658. [Google Scholar] [CrossRef]
- Nagasawa, K. The Biology of Contracaecum Osculatum Sensu Lato and C. Osculatum A (Nematoda: Anisakidae) in Japanese Waters: A Review. Biosph. Sci. 2012, 51, 61–69. [Google Scholar]
- Abdelrahman, T.; Alsaeed, M.; Karam, R.; Alkhthami, A.; Alswat, O.; Alzahrani, A.; Hendi, O.; Jawad, H. Misuse of Antibiotics and Antibiotic Resistance: A Public Population-Based Health Survey in Altaif-Saudi Arabia. WJPMR 2017, 2, 54–62. [Google Scholar]
- Yoshizaki, G.; Yazawa, R. Application of Surrogate Broodstock Technology in Aquaculture. Fish. Sci. 2019, 85, 429–437. [Google Scholar] [CrossRef]
- Lee, S.; Iwasaki, Y.; Yoshizaki, G. Long-Term (5 Years) Cryopreserved Spermatogonia Have High Capacity to Generate Functional Gametes via Interspecies Transplantation in Salmonids. Cryobiology 2016, 73, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Weber, G.M.; Lee, C.-S. Current and Future Assisted Reproductive Technologies for Fish Species. In Current and Future Reproductive Technologies and World Food Production; Lamb, G.C., DiLorenzo, N., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2014; Volume 752, pp. 33–76. ISBN 978-1-4614-8886-6. [Google Scholar]
- Mohanty, B.P.; Ganguly, S.; Mahanty, A.; Mitra, T.; Mohanty, S. Nutrigenomics and Fish. CABI Rev. 2020, 2020, PAVSNNR202015048. [Google Scholar] [CrossRef]
- Dawson, K.A. Nutrigenomics: Feeding the Genes for Improved Fertility. Anim. Reprod. Sci. 2006, 96, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Overturf, K.; Kajbaf, K. Nutrigenomic Approaches Improve the Efficiency of Soybean Meal Utilization in Salmonids Aquaculture. Av. Nutr. Acuicola 2022, 1, 156–157. [Google Scholar]
- Tincy, V.; Mishal, P.; Akhtar, M.S.; Pal, A.K. Turning Challenges into Opportunities. World Aquac. 2014, 45, 67–69. [Google Scholar]





| Drivers of Change | Impacts |
|---|---|
| Variation of the surface temperature of the waters | Expansion of harmful algae Lowering of dissolved O2 levels Spread of disease and parasites Extension of growth stations Changes of position and ranges of suitable species Mortality decreases during the winter season Enhanced growth and food conversion rates Alteration of local ecosystems induced by competition, parasitism and predation of competitors and exotic species |
| Variation of oceanographic variables | Decreased flushing rates and food availability to shellfish Alteration in the richness of edible species and fishmeal |
| Sea level rise | Reduction of areas destined for aquaculture Reduction of areas that provide physical protection Increased risks of flooding Salt infiltration into groundwater |
| Increased frequency of storms | Larger waves More frequent storms Floods caused by rainfall Variation of salinity parameters Structure damage |
| Drought and water stress | Variation of salinity parameters Decrease in water quality Increased disease Uncertain water supplies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitra, A.; Abdel-Gawad, F.K.; Bassem, S.; Barua, P.; Assisi, L.; Parisi, C.; Temraz, T.A.; Vangone, R.; Kajbaf, K.; Kumar, V.; et al. Climate Change and Reproductive Biocomplexity in Fishes: Innovative Management Approaches towards Sustainability of Fisheries and Aquaculture. Water 2023, 15, 725. https://doi.org/10.3390/w15040725
Mitra A, Abdel-Gawad FK, Bassem S, Barua P, Assisi L, Parisi C, Temraz TA, Vangone R, Kajbaf K, Kumar V, et al. Climate Change and Reproductive Biocomplexity in Fishes: Innovative Management Approaches towards Sustainability of Fisheries and Aquaculture. Water. 2023; 15(4):725. https://doi.org/10.3390/w15040725
Chicago/Turabian StyleMitra, Anisa, Fagr Kh. Abdel-Gawad, Samah Bassem, Prabal Barua, Loredana Assisi, Costantino Parisi, Tarek A. Temraz, Rubina Vangone, Kimia Kajbaf, Vikas Kumar, and et al. 2023. "Climate Change and Reproductive Biocomplexity in Fishes: Innovative Management Approaches towards Sustainability of Fisheries and Aquaculture" Water 15, no. 4: 725. https://doi.org/10.3390/w15040725