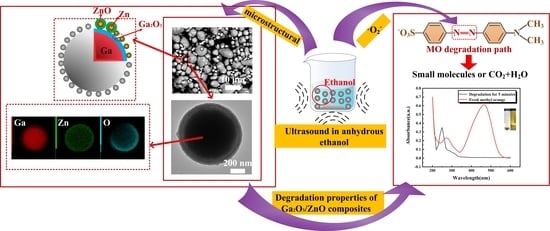
Design of a Spherical Ga2O3/ZnO Composite with a Snakeberry-like Structure for Methyl Orange Degradation
Abstract
:1. Introduction
2. Experiment
2.1. Synthesis Method
2.2. Degradation Experiment
2.3. Characterization of Microstructure
3. Results and Discussion
3.1. XRD
3.2. SEM and TEM
3.3. XPS
3.4. Brunauer–Emmett–Teller (BET)
4. Catalytic Performance of the Ga–Based Liquid Metal
4.1. Degradation Performance
4.2. Influencing Factors
5. Analysis of the Degradation Principle
5.1. Free Radical Capture Experiment
5.2. Analysis of the Degradation Process
6. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Shi, R.; Luo, H.; Zhang, R.; Hu, Y.; Xie, H.; Zhu, N.-M. Alkali-catalyzed hydrothermal oxidation treatment of triclosan in soil: Mechanism, degradation pathway and toxicity evaluation. Sci. Total Environ. 2023, 856, 159187. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Shao, Y.; Ji, W.; Zeng, Y.; Xu, J.; Liu, W.; Xu, L.; Wu, D. Trace Mn (II)-catalyzed periodate oxidation of organic contaminants not relying on any transient reactive species: The substrate-dependent dual roles of in-situ formed colloidal MnO2. Chem. Eng. J. 2023, 451, 139106. [Google Scholar] [CrossRef]
- Abdallah, A.E.M. Recent green synthesis of pyridines and their fused systems catalyzed by nanocatalysts. In Recent Developments in the Synthesis and Applications of Pyridines; Elsevier: Amsterdam, The Netherlands, 2023; pp. 331–375. [Google Scholar]
- Dihom, H.R.; Al-Shaibani, M.M.; Mohamed, R.M.S.R.; Al-Gheethi, A.A.; Sharma, A.; Khamidun, M.H.B. Photocatalytic degradation of disperse azo dyes in textile wastewater using green zinc oxide nanoparticles synthesized in plant extract: A critical review. J. Water Process Eng. 2022, 47, 102705. [Google Scholar] [CrossRef]
- Tu, Y.; Shao, G.; Zhang, W.; Chen, J.; Qu, Y.; Zhang, F.; Tian, S.; Zhou, Z.; Ren, Z. The degradation of printing and dyeing wastewater by manganese-based catalysts. Sci. Total Environ. 2022, 828, 154390. [Google Scholar] [CrossRef]
- Singh, B.; Singh, P.; Siddiqui, S.; Singh, D.; Gupta, M. Wastewater treatment using Fe-doped perovskite manganites by photocatalytic degradation of methyl orange, crystal violet and indigo carmine dyes in tungsten bulb/sunlight. J. Rare Earths 2022, in press. [CrossRef]
- Sobhanardakani, S.; Zandipak, R.; Khoshsafar, H. Removal of cationic dyes from aqueous solutions using NiFe2O4 nanoparticles. J. Water Supply Res. Technol. AQUA 2016, 65, 64–74. [Google Scholar]
- Mahmoodi, N.M.; Taghizadeh, A.; Taghizadeh, M.; Baglou, M.A.S. Surface modified montmorillonite with cationic surfactants: Preparation, characterization, and dye adsorption from aqueous solution. J. Environ. Chem. Eng. 2019, 7, 103243. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, J.; Ren, Y.; Yang, Z.; Zhong, M.; Feng, X.; Su, B.; Lei, Z. Excellent adsorptive-photocatalytic performance of zinc oxide and biomass derived N, O-contained biochar nanocomposites for dyes and antibiotic removal. Chem. Eng. J. 2023, 451, 138959. [Google Scholar] [CrossRef]
- Sun, H.; Lee, S.-Y.; Park, S.-J. Bimetallic CuPd alloy nanoparticles decorated ZnO nanosheets with enhanced photocatalytic degradation of methyl orange dye. J. Colloid Interface Sci. 2023, 629, 87–96. [Google Scholar] [CrossRef]
- Chi, Z.; Tarntair, F.-G.; Frégnaux, M.; Wu, W.-Y.; Sartel, C.; Madaci, I.; Chapon, P.; Sallet, V.; Dumont, Y.; Pérez-Tomás, A.; et al. Bipolar self-doping in ultra-wide bandgap spinel ZnGa2O4. Mater. Today Phys. 2021, 20, 100466. [Google Scholar] [CrossRef]
- Liang, J.; Chai, Y.; Li, L.; Li, D.; Shen, J.; Zhang, Y.; Wang, X. Germanium and iron double-substituted ZnGa2O4 solid-solution photocatalysts with modulated band structure for boosting photocatalytic CO2 reduction with H2O. Appl. Catal. B Environ. 2020, 265, 118551. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, J.; Ding, K.; Hou, Y.; Wang, X.; Fu, X. Photocatalytic decomposition of benzene by porous nanocrystalline ZnGa2O4 with a high surface area. Environ. Sci. Technol. 2009, 43, 5947–5951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, J.; Chen, Z.; Wang, T. Photocatalytic degradation of methylene blue by ZnGa2O4 thin films. Catal. Commun. 2009, 10, 1781–1785. [Google Scholar] [CrossRef]
- Liu, L.; Huang, J.; Cao, L.; Wu, J.; Fei, J.; Ouyang, H.; Ma, F.; Zhou, C. Synthesis of ZnGa2O4 octahedral crystallite by hydrothermal method with the aid of CTAB and its photocatalytic activity. Mater. Lett. 2013, 95, 160–163. [Google Scholar] [CrossRef]
- Liu, J.; Lu, W.; Wu, H.; Jin, L.; Hu, B.; Li, L.; Wang, Z. In situ synthesis of rice-like ZnGa2O4 for the photocatalytic removal of organic and inorganic pollutants. Mater. Sci. Semicond. Process. 2016, 56, 251–259. [Google Scholar] [CrossRef]
- Liu, L.L.; Cao, L.-Y.; Huang, J.-H.; Zhang, X.-W. Preparation and Photocatalytic Properties of the Octahedral ZnGa2O4 Crystallites. Chin. J. Inorg. Chem. 2012, 28, 2091–2096. [Google Scholar]
- Yuan, Y.; Huang, J.; Tu, W.; Huang, S. Synthesis of uniform ZnGa2O4 nanoparticles with high photocatalytic activity. J. Alloys Compd. 2014, 616, 461–467. [Google Scholar] [CrossRef]
- Negrete-Durán, S.; Villabona-Leal, E.; Alanis, J.; Rodríguez-Aranda, M.; Ojeda-Galván, H.J.; Hernández-Arteaga, A.; Ovando, V.; Cardoso, P.; Quintana, M.; Ocampo-Pérez, R.; et al. Thermal evolution of Zn3(OH)2V2O7·2H2O to Zn-VO family (Zn2(OH)(VO4), Zn3(VO4)2-α, ZnO and Zn3(VO4)2-β): A structural, optical and visible light photocatalytic study. J. Alloys Compd. 2023, 932, 167493. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J.; Nan, J.; Gai, C.; Shao, Q.; Murugadoss, V.; Maganti, S.; Naik, N.; Algadi, H.; Huang, M.; et al. Influence of mass ratio and calcination temperature on physical and photoelectrochemical properties of ZnFe-layered double oxide/cobalt oxide heterojunction semiconductor for dye degradation applications. Particuology 2023, 74, 141–155. [Google Scholar] [CrossRef]
- Sampath, S.; Dharmar, S.; Chinnasamy, K.; Bangaru, G.; Sankar, M.; Gedi, S.; Shkir, M.; Manthrammel, M.A. Improved ethanol sensing and photocatalytic Rhodamine B dye degradation of Ni-SnO2 nanoparticles. Mater. Sci. Eng. B 2023, 287, 116091. [Google Scholar] [CrossRef]
- Ren, Z.; Yang, X.; Zhang, W.; Zhao, Z. Preparation, characterization and performance of a novel magnetic Fe-Zn activated carbon for efficient removal of dyes from wastewater. J. Mol. Struct. 2023, 1274, 134407. [Google Scholar] [CrossRef]
- Oloye, O.; Riches, J.D.; O’Mullane, A.P. Liquid metal assisted sonocatalytic degradation of organic azo dyes to solid carbon particles. Chem. Commun. 2021, 57, 9296–9299. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.; Yang, W.; Zhu, M.; Shi, J. The improvement of coralline-like ZnGa2O4 by cocatalysts for the photocatalytic degradation of rhodamine B. Catalysts 2020, 10, 221. [Google Scholar] [CrossRef] [Green Version]
- Hohman, J.N.; Kim, M.; Wadsworth, G.A.; Bednar, H.R.; Jiang, J.; LeThai, M.A.; Weiss, P.S. Directing substrate morphology via self-assembly: Ligand-mediated scission of gallium–indium microspheres to the nanoscale. Nano Lett. 2011, 11, 5104–5110. [Google Scholar] [CrossRef]
- Hu, L.; Yuan, B.; Liu, J. Liquid metal amoeba with spontaneous pseudopodia formation and motion capability. Sci. Rep. 2017, 7, 7256. [Google Scholar] [CrossRef] [Green Version]
- Cui, S.; Li, J.; Zhang, L.; Jiang, Y.; Wang, Z.; Zhang, L.; Yuan, C.; Shen, Z.; Zeng, H. Effect of Ga2O3-doping on Properties and Structure of ZBLAN Glass. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2022, 37, 564–569. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, Y.; Genzer, J.; Dickey, M.D. Shape-transformable liquid metal nanoparticles in aqueous solution. Chem. Sci. 2017, 8, 3832–3837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Chen, Y.; Ren, F.-F.; Gu, S.L.; Tan, H.H.; Jagadish, C.; Ye, J.D. Band alignment and band bending at α-Ga2O3/ZnO nn isotype hetero-interface. Appl. Phys. Lett. 2019, 115, 202101. [Google Scholar] [CrossRef]
- Parida, K.; Martha, S.; Das, D.; Biswal, N. Facile fabrication of hierarchical N-doped GaZn mixed oxides for water splitting reactions. J. Mater. Chem. 2010, 20, 7144–7149. [Google Scholar] [CrossRef]
- Chen, Y.; Ning, H.; Kuang, Y.; Yu, X.-X.; Gong, H.-H.; Chen, X.; Ren, F.-F.; Gu, S.; Zhang, R.; Zheng, Y.; et al. Band alignment and polarization engineering in κ-Ga2O3/GaN ferroelectric heterojunction. Sci. China Phys. Mech. Astron. 2022, 65, 277311. [Google Scholar] [CrossRef]
- Gong, H.; Chen, X.; Xu, Y.; Chen, Y.; Ren, F.-F.; Liu, B.; Gu, S.; Zhang, R.; Ye, J. Band alignment and interface recombination in NiO/β-Ga2O3 Type-II pn heterojunctions. IEEE Trans. Electron Devices 2020, 67, 3341–3347. [Google Scholar] [CrossRef]
- Navarro-Quezada, A.; Galazka, Z.; Alamé, S.; Skuridina, D.; Vogt, P.; Esser, N. Surface properties of annealed semiconducting β-Ga2O3 (100) single crystals for epitaxy. Appl. Surf. Sci. 2015, 349, 368–373. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef]
- Shah, M.S.A.S.; Zhang, K.; Park, A.R.; Kim, K.S.; Park, N.-G.; Parkab, J.H.; Yoo, P.J. Single-step solvothermal synthesis of mesoporous Ag–TiO2–reduced graphene oxide ternary composites with enhanced photocatalytic activity. Nanoscale 2013, 5, 5093–50101. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Li, Y.; Wang, X.; Long, J.; Liang, B. Carbon nanosheet/MnO2/BiOCl ternary composite for degradation of organic pollutants. J. Alloys Compd. 2022, 891, 162090. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, C.; Tang, S.; Li, X.; Tang, J.; Rao, Y.; Wang, Z.; Zhang, Q. Enhancing CaO2 fenton-like process by Fe (II)-oxalic acid complexation for organic wastewater treatment. Water Res. 2019, 163, 114861. [Google Scholar] [CrossRef] [PubMed]
- Nathiya, D.; Alhaji, N.; Jahangir, A.M.; Fathima, M.I.; Gatasheh, M.K.; Hatamleh, A.A.; Zehra, S.; Ayeshamariam, A. Synthesis and characterization of ZnGa2O4 composites and its photocatalytic properties for energy applications. Environ. Res. 2022, 204, 112073. [Google Scholar] [CrossRef]
- Lu, H.-S.; Zhang, H.; Liu, R.; Zhang, X.; Zhao, H.; Wang, G. Macroscale cobalt-MOFs derived metallic Co nanoparticles embedded in N-doped porous carbon layers as efficient oxygen electrocatalysts. Appl. Surf. Sci. 2017, 392, 402–409. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Duan, L.; Zhang, D. Decolorization of methyl orange by ozonation in combination with ultrasonic irradiation. J. Hazard. Mater. 2006, 138, 53–59. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Liu, Y.; Fan, Y.; Dang, F.; Qiu, Y.; Zhou, H.; Wang, W.; Liu, Y. Solvothermal Synthesis of ZnO Nanoparticles for Photocatalytic Degradation of Methyl Orange and p-Nitrophenol. Water 2021, 13, 3224. [Google Scholar] [CrossRef]
- Wang, C.; Qin, H.; Cao, L.; Wang, D.; Zhang, J.; Zhang, B.; Ou, X. Engineered single-crystal metal-selenide for rapid K-ion diffusion and polyselenide convention. Chem. Eng. J. 2022, 427, 131963. [Google Scholar] [CrossRef]
- Hong, X.; Wang, R.; Li, S.; Fu, J.; Chen, L.; Wang, X. Hydrophilic macroporous SnO2/rGO composite prepared by melamine template for high efficient photocatalyst. J. Alloys Compd. 2020, 816, 152550. [Google Scholar] [CrossRef]
- Chen, W.; Sun, F.; Zhu, Z.; Min, Z.; Li, W. Nanoporous SnO2 prepared by a photochemical strategy: Controlling of specific surface area and photocatalytic activity in degradation of dye pollutants. Microporous Mesoporous Mater. 2014, 186, 65–72. [Google Scholar] [CrossRef]
- Shang, J.; Chen, T.; Wang, X.; Sun, L.; Su, Q. Facile fabrication and enhanced photocatalytic performance: From BiOCl to element-doped BiOCl. Chem. Phys. Lett. 2018, 706, 483–487. [Google Scholar] [CrossRef]
- Mohammed, M.G.; Dickey, M.D. Strain-controlled diffraction of light from stretchable liquid metal micro-components. Sens. Actuators A Phys. 2013, 193, 246–250. [Google Scholar] [CrossRef]
- Liang, C.; Su, H.-W. Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Liu, J.; Dong, Y.; Zhang, L.; Liu, W.; Zhang, C.; Shi, Y.; Lin, H. Regulating superoxide radicals and light absorption ability for enhancing photocatalytic performance of MoS2@ Z by CeO2 rich in adsorbed oxygen. J. Clean. Prod. 2021, 322, 129059. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, Y.; Li, Q.; Zhang, Q.; Zhang, B. Fabrication of folded MXene/MoS2 composite microspheres with optimal composition and their microwave absorbing properties. J. Colloid Interface Sci. 2022, 607, 633–644. [Google Scholar] [CrossRef]
- Chen, S.; Feng, W.; Wang, H.; Wu, Z. Synergistic degradation of NO and ethyl acetate by plasma activated “pseudo photocatalysis” on Ce/ZnGa2O4/NH2-UiO-66 catalyst: Restrictive relation and reaction pathways exploration. Chem. Eng. J. 2021, 421, 129725. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; Wang, X.; Hong, X. Construction of a hydrangea-like Bi2WO6/BiOCl composite as a high-performance photocatalyst. New J. Chem. 2022, 46, 2627–2634. [Google Scholar] [CrossRef]
- Hunge, Y.; Mahadik, M.; Moholkar, A.; Bhosale, C.H. Photoelectrocatalytic degradation of phthalic acid using spray deposited stratified WO3/ZnO thin films under sunlight illumination. Appl. Surf. Sci. 2017, 420, 764–772. [Google Scholar] [CrossRef]
- Méndez-Díaz, J.D.; Rivera-Utrilla, J.; Sánchez-Polo, M.; Bautista-Toledo, I. Adsorption/bioadsorption of phthalic acid, an organic micropollutant present in landfill leachates, on activated carbons. J. Colloid Interface Sci. 2012, 369, 358–365. [Google Scholar] [CrossRef]
- Mageshwari, K.; Nataraj, D.; Pal, T.; Sathyamoorthy, R.; Park, J. Improved photocatalytic activity of ZnO coupled CuO nanocomposites synthesized by reflux condensation method. J. Alloys Compd. 2015, 625, 362–370. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, C.; Chen, S.; Fu, X. Ag3PO4/ZnO: An efficient visible-light-sensitized composite with its application in photocatalytic degradation of Rhodamine B. Mater. Res. Bull. 2013, 48, 106–113. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, C.; Chen, S.; Fu, X. Significantly enhanced visible-light photocatalytic activity of g-C3N4 via ZnO modification and the mechanism study. J. Mol. Catal. A Chem. 2013, 368, 9–15. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y.J. Synthesis of M@TiO2(M= Au, Pd, Pt) core–shell nanocomposites with tunable photoreactivity. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]
- Katsumata, H.; Oda, Y.; Kaneco, S.; Suzuki, T. Photocatalytic activity of Ag/CuO/WO3 under visible-light irradiation. RSC Adv. 2013, 3, 5028–5035. [Google Scholar] [CrossRef]
- Abe, R.; Takami, H.; Murakami, N.; Otani, B. Pristine simple oxides as visible light driven photocatalysts: Highly efficient decomposition of organic compounds over platinum-loaded tungsten oxide. J. Am. Chem. Soc. 2008, 130, 7780–7781. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Yang, W.; Chen, X.; Li, W.; Luo, B.; Wang, K. Preparation of 3D PbO2 nanospheres@ SnO2 nanowires/Ti electrode and its application in methyl orange degradation. Electrochim. Acta 2014, 146, 15–22. [Google Scholar] [CrossRef]










| Sample | Specific Surface Area/(m2/g) | Average Aperture /(nm) | Pore Volume /(mL/g) |
|---|---|---|---|
| Ga | 0.462 | 30.987 | 0.003 |
| Ga2O3/ZnO | 0.868 | 29.197 | 0.002 |
| Element | Fresh Weight Ratio | Element | Used Weight Ratio |
|---|---|---|---|
| Zn | 3.5 | Zn | 3.19 |
| Ga | 96.5 | Ga | 96.81 |
| total | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Liu, G.; Chen, Z.; Xu, X.; Wei, C. Design of a Spherical Ga2O3/ZnO Composite with a Snakeberry-like Structure for Methyl Orange Degradation. Water 2023, 15, 952. https://doi.org/10.3390/w15050952
Xie H, Liu G, Chen Z, Xu X, Wei C. Design of a Spherical Ga2O3/ZnO Composite with a Snakeberry-like Structure for Methyl Orange Degradation. Water. 2023; 15(5):952. https://doi.org/10.3390/w15050952
Chicago/Turabian StyleXie, Hongyu, Guangzhu Liu, Zelin Chen, Xintong Xu, and Chong Wei. 2023. "Design of a Spherical Ga2O3/ZnO Composite with a Snakeberry-like Structure for Methyl Orange Degradation" Water 15, no. 5: 952. https://doi.org/10.3390/w15050952