Continuous-Flow Aerobic Granular Sludge Treatment of Dairy Wastewater
Abstract
:1. Introduction
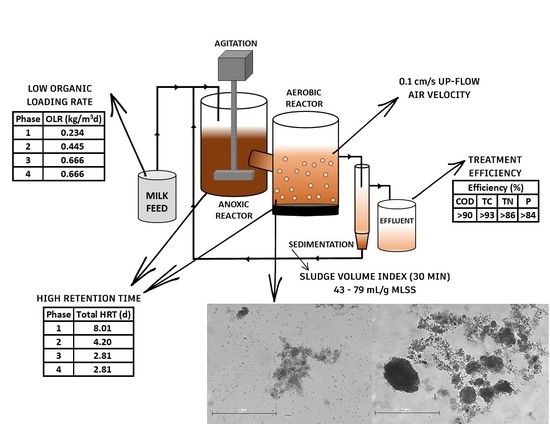
2. Materials and Methods
2.1. Influent
2.2. Inoculum
2.3. Image Analysis
2.4. Solids and SVI Measurements
2.5. Analytical Methods
2.6. Setup
2.7. Operating Strategy
3. Results and Discussion
3.1. Sludge Characteristics and Development of AGS
3.2. Performance of the CFR System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stasinakis, A.S.; Charalambous, P.; Vyrides, I. Dairy Wastewater Management in EU: Produced Amounts, Existing Legislation, Applied Treatment Processes and Future Challenges. J. Environ. Manag. 2022, 303, 114152. [Google Scholar] [CrossRef]
- FAO. Dairy Market Review. Available online: https://www.fao.org/3/cc1189en/cc1189en.pdf (accessed on 18 October 2022).
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Rahman, U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and Utilization of Dairy Industrial Waste: A Review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Porwal, H.J.; Mane, A.V.; Velhal, S.G. Biodegradation of Dairy Effluent by Using Microbial Isolates Obtained from Activated Sludge. Water Resour. Ind. 2015, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Struk-Sokoowska, J.; Rodziewicz, J.; Mielcarek, A. Effect of Dairy Wastewater on Changes in COD Fractions in Technical-Scale SBR Type Reactors. Water Sci. Technol. 2018, 2017, 156–169. [Google Scholar] [CrossRef]
- Bella, K.; Rao, P.V. Anaerobic Digestion of Dairy Wastewater: Effect of Different Parameters and Co-Digestion Options—A Review. Biomass Convers. Biorefinery 2021, 13, 2527–2552. [Google Scholar] [CrossRef]
- Demirel, B.; Yenigun, O.; Onay, T.T. Anaerobic Treatment of Dairy Wastewaters: A Review. Process Biochem. 2005, 40, 2583–2595. [Google Scholar] [CrossRef]
- Skoczko, I.; Struk-Sokołowska, J.; Ofman, P. Seasonal Changes in Nitrogen, Phosphorus, Bod and Cod Removal in Bystre Wastewater Treatment Plant. J. Ecol. Eng. 2017, 18, 185–191. [Google Scholar] [CrossRef]
- Miito, G.J.; Ndegwa, P.; Alege, F.P.; Coulibaly, S.S.; Davis, R.; Harrison, J. A Vermifilter System for Reducing Nutrients and Organic-Strength of Dairy Wastewater. Environ. Technol. Innov. 2021, 23, 101648. [Google Scholar] [CrossRef]
- Kolev Slavov, A. Dairy Wastewaters—General Characteristics and Treatment Possibilities—A Review. Food Technol. Biotechnol. 2017, 55, 14. [Google Scholar] [CrossRef]
- Lefèbvre, B. (Ed.) The Activated Sludge Sludge Process; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2019; ISBN 978-1-53615-202-9. [Google Scholar]
- Wilén, B.M.; Liébana, R.; Persson, F.; Modin, O.; Hermansson, M. The Mechanisms of Granulation of Activated Sludge in Wastewater Treatment, Its Optimization, and Impact on Effluent Quality. Appl. Microbiol. Biotechnol. 2018, 102, 5005–5020. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, J.A.; Almeida-Naranjo, C.E.; Villamar Ayala, C.A. Improvement of Nutrients Removal from Domestic Wastewater by Activated-Sludge Encapsulation with Polyvinyl Alcohol (PVA). J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2019, 54, 721–727. [Google Scholar] [CrossRef]
- de Sousa Rollemberg, S.L.; Mendes Barros, A.R.; Milen Firmino, P.I.; dos Santos, A.B. Aerobic Granular Sludge: Cultivation Parameters and Removal Mechanisms. Bioresour. Technol. 2018, 270, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Sarvajith, M. Aerobic Granular Sludge Process: A Fast Growing Biological Treatment for Sustainable Wastewater Treatment. Curr. Opin. Environ. Sci. Health 2019, 12, 57–65. [Google Scholar] [CrossRef]
- Thwaites, B.J.; Short, M.D.; Stuetz, R.M.; Reeve, P.J.; Alvarez Gaitan, J.P.; Dinesh, N.; van den Akker, B. Comparing the Performance of Aerobic Granular Sludge versus Conventional Activated Sludge for Microbial Log Removal and Effluent Quality: Implications for Water Reuse. Water Res. 2018, 145, 442–452. [Google Scholar] [CrossRef]
- Lemaire, R.; Webb, R.I.; Yuan, Z. Micro-Scale Observations of the Structure of Aerobic Microbial Granules Used for the Treatment of Nutrient-Rich Industrial Wastewater. ISME J. 2008, 2, 528–541. [Google Scholar] [CrossRef] [Green Version]
- Rosa-Masegosa, A.; Muñoz-Palazon, B.; Gonzalez-Martinez, A.; Fenice, M.; Gorrasi, S.; Gonzalez-Lopez, J. New Advances in Aerobic Granular Sludge Technology Using Continuous Flow Reactors: Engineering and Microbiological Aspects. Water 2021, 13, 1792. [Google Scholar] [CrossRef]
- de Kreuk, M.K.; Kishida, N.; van Loosdrecht, M.C.M. Aerobic Granular Sludge—State of the Art. Water Sci. Technol. 2007, 55, 75–81. [Google Scholar] [CrossRef]
- Franca, R.D.G.; Pinheiro, H.M.; van Loosdrecht, M.C.M.; Lourenço, N.D. Stability of Aerobic Granules during Long-Term Bioreactor Operation. Biotechnol. Adv. 2018, 36, 228–246. [Google Scholar] [CrossRef]
- Linlin, H.; Jianlong, W.; Xianghua, W.; Yi, Q. The Formation and Characteristics of Aerobic Granules in Sequencing Batch Reactor (SBR) by Seeding Anaerobic Granules. Process Biochem. 2005, 40, 5–11. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H. Aerobic Granulation: Advances and Challenges. Appl. Biochem. Biotechnol. 2012, 167, 1622–1640. [Google Scholar] [CrossRef]
- Kent, T.R.; Bott, C.B.; Wang, Z.W. State of the Art of Aerobic Granulation in Continuous Flow Bioreactors. Biotechnol. Adv. 2018, 36, 1139–1166. [Google Scholar] [CrossRef]
- Xu, D.; Li, J.; Liu, J.; Qu, X.; Ma, H. Advances in Continuous Flow Aerobic Granular Sludge: A Review. Process Saf. Environ. Prot. 2022, 163, 27–35. [Google Scholar] [CrossRef]
- Hou, Y.; Gan, C.; Chen, R.; Chen, Y.; Yuan, S.; Chen, Y. Structural Characteristics of Aerobic Granular Sludge and Factors That Influence Its Stability: A Mini Review. Water 2021, 13, 2726. [Google Scholar] [CrossRef]
- Morgenroth, E.; Sherden, T.; Van Loosdrecht, M.C.M.; Heijnen, J.J.; Wilderer, P.A. Aerobic Granular Sludge in a Sequencing Batch Reactor. Water Res. 1997, 31, 3191–3194. [Google Scholar] [CrossRef]
- Gao, D.; Liu, L.; Liang, H.; Wu, W.M. Aerobic Granular Sludge: Characterization, Mechanism of Granulation and Application to Wastewater Treatment. Crit. Rev. Biotechnol. 2011, 31, 137–152. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Zaghloul, M.S.; Hamza, R.A.; Tay, J.H. Long-Term Aerobic Granular Sludge Stability through Anaerobic Slow Feeding, Fixed Feast-Famine Period Ratio, and Fixed SRT. J. Environ. Chem. Eng. 2020, 8, 103681. [Google Scholar] [CrossRef]
- Figdore, B.A.; Stensel, H.D.; Winkler, M.K.H. Comparison of Different Aerobic Granular Sludge Types for Activated Sludge Nitrification Bioaugmentation Potential. Bioresour. Technol. 2018, 251, 189–196. [Google Scholar] [CrossRef]
- Wei, S.P.; Stensel, H.D.; Ziels, R.M.; Herrera, S.; Lee, P.H.; Winkler, M.K.H. Partitioning of Nutrient Removal Contribution between Granules and Flocs in a Hybrid Granular Activated Sludge System. Water Res. 2021, 203, 117514. [Google Scholar] [CrossRef]
- Lochmatter, S.; Holliger, C. Optimization of Operation Conditions for the Startup of Aerobic Granular Sludge Reactors Biologically Removing Carbon, Nitrogen, and Phosphorous. Water Res. 2014, 59, 58–70. [Google Scholar] [CrossRef]
- de Sousa Rollemberg, S.L.; Ferreira, T.J.T.; Firmino, P.I.M.; dos Santos, A.B. Impact of Cycle Type on Aerobic Granular Sludge Formation, Stability, Removal Mechanisms and System Performance. J. Environ. Manag. 2020, 256, 109970. [Google Scholar] [CrossRef]
- de Kreuk, M.K.; Heijnen, J.J.; van Loosdrecht, M.C.M. Simultaneous COD, Nitrogen, and Phosphate Removal by Aerobic Granular Sludge. Biotechnol. Bioeng. 2005, 90, 761–769. [Google Scholar] [CrossRef]
- de Kreuk, M.K.; Kishida, N.; Tsuneda, S.; van Loosdrecht, M.C.M. Behavior of Polymeric Substrates in an Aerobic Granular Sludge System. Water Res. 2010, 44, 5929–5938. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Gabus, S.; Rohrbach-Brandt, E.; Hosseini, M.; Rossi, P.; Maillard, J.; Holliger, C. Performance and Microbial Community Composition Dynamics of Aerobic Granular Sludge from Sequencing Batch Bubble Column Reactors Operated at 20 °C, 30 °C, and 35 °C. Appl. Microbiol. Biotechnol. 2010, 87, 1555–1568. [Google Scholar] [CrossRef] [Green Version]
- Jahn, L.; Saracevic, E.; Svardal, K.; Krampe, J. Anaerobic Biodegradation and Dewaterability of Aerobic Granular Sludge. J. Chem. Technol. Biotechnol. 2019, 94, 2908–2916. [Google Scholar] [CrossRef] [Green Version]
- Adler, A.; Holliger, C. Multistability and Reversibility of Aerobic Granular Sludge Microbial Communities Upon Changes From Simple to Complex Synthetic Wastewater and Back. Front. Microbiol. 2020, 11, 574361. [Google Scholar] [CrossRef]
- De Vleeschauwer, F.; Caluwé, M.; Dobbeleers, T.; Stes, H.; Dockx, L.; Kiekens, F.; Copot, C.; Dries, J. A Dynamically Controlled Anaerobic/Aerobic Granular Sludge Reactor Efficiently Treats Brewery/Bottling Wastewater. Water Sci. Technol. 2021, 84, 3515–3527. [Google Scholar] [CrossRef]
- Zou, J.; Tao, Y.; Li, J.; Wu, S.; Ni, Y. Cultivating Aerobic Granular Sludge in a Developed Continuous-Flow Reactor with Two-Zone Sedimentation Tank Treating Real and Low-Strength Wastewater. Bioresour. Technol. 2018, 247, 776–783. [Google Scholar] [CrossRef]
- Devlin, T.R.; Oleszkiewicz, J.A. Design of Aerobic Granular Sludge in Continuous Flow Reactors. U.S. Patent US 17/043,248, 18 November 2021. [Google Scholar]
- Juang, Y.C.; Adav, S.S.; Lee, D.J.; Tay, J.H. Stable Aerobic Granules for Continuous-Flow Reactors: Precipitating Calcium and Iron Salts in Granular Interiors. Bioresour. Technol. 2010, 101, 8051–8057. [Google Scholar] [CrossRef]
- Long, B.; Yang, C.Z.; Pu, W.H.; Yang, J.K.; Liu, F.B.; Zhang, L.; Cheng, K. Rapid Cultivation of Aerobic Granular Sludge in a Continuous Flow Reactor. J. Environ. Chem. Eng. 2015, 3, 2966–2973. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Li, S.; Zeng, H.; Zhang, J. Aerobic Granular Sludge Operation and Nutrients Removal Mechanism in a Novel Configuration Reactor Combined Sequencing Batch Reactor and Continuous-Flow Reactor. Bioresour. Technol. 2019, 292, 122024. [Google Scholar] [CrossRef]
- Li, S.; Li, D.; Wang, Y.; Zeng, H.; Yuan, Y.; Zhang, J. Startup and Stable Operation of Advanced Continuous Flow Reactor and the Changes of Microbial Communities in Aerobic Granular Sludge. Chemosphere 2020, 243, 125434. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.L.; Cui, Y.W.; Huang, J.L. Continuous Flow Reactors for Cultivating Aerobic Granular Sludge: Configuration Innovation, Principle and Research Prospect. J. Chem. Technol. Biotechnol. 2021, 96, 2721–2734. [Google Scholar] [CrossRef]
- Jahn, L.; Svardal, K.; Krampe, J. Comparison of Aerobic Granulation in SBR and Continuous-Flow Plants. J. Environ. Manag. 2019, 231, 953–961. [Google Scholar] [CrossRef]
- Strubbe, L.; Pennewaerde, M.; Baeten, J.E.; Volcke, E.I.P. Continuous Aerobic Granular Sludge Plants: Better Settling versus Diffusion Limitation. Chem. Eng. J. 2022, 428, 131427. [Google Scholar] [CrossRef]
- Ahmad, J.S.M.; Cai, W.; Zhao, Z.; Zhang, Z.; Shimizu, K.; Lei, Z.; Lee, D.J. Stability of Algal-Bacterial Granules in Continuous-Flow Reactors to Treat Varying Strength Domestic Wastewater. Bioresour. Technol. 2017, 244, 225–233. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Yang, C.; Pu, W.; He, L.; Bo, F. Stable Aerobic Granules in Continuous-Flow Bioreactor with Self-Forming Dynamic Membrane. Bioresour. Technol. 2012, 121, 111–118. [Google Scholar] [CrossRef]
- Devlin, T.R.; Oleszkiewicz, J.A. Cultivation of Aerobic Granular Sludge in Continuous Flow under Various Selective Pressure. Bioresour. Technol. 2018, 253, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, A.; Ding, L.; Sellamuthu, B.; Perreault, J. Aerobic Sludge Granulation in a Reverse Flow Baffled Reactor (RFBR) Operated in Continuous-Flow Mode for Wastewater Treatment. Sep. Purif. Technol. 2015, 149, 437–444. [Google Scholar] [CrossRef]
- Zhou, D.; Dong, S.; Gao, L.; Liu, M.; Niu, S. Distribution Characteristics of Extracellular Polymeric Substances and Cells of Aerobic Granules Cultivated in a Continuous-Flow Airlift Reactor. J. Chem. Technol. Biotechnol. 2013, 88, 942–947. [Google Scholar] [CrossRef]
- Tsuneda, S.; Nagano, T.; Hoshino, T.; Ejiri, Y.; Noda, N.; Hirata, A. Characterization of Nitrifying Granules Produced in an Aerobic Upflow Fluidized Bed Reactor. Water Res. 2003, 37, 4965–4973. [Google Scholar] [CrossRef]
- Li, D.; Lv, Y.; Zeng, H.; Zhang, J. Startup and Long Term Operation of Enhanced Biological Phosphorus Removal in Continuous-Flow Reactor with Granules. Bioresour. Technol. 2016, 212, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Corsino, S.F.; Campo, R.; Di Bella, G.; Torregrossa, M.; Viviani, G. Study of Aerobic Granular Sludge Stability in a Continuous-Flow Membrane Bioreactor. Bioresour. Technol. 2016, 200, 1055–1059. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Palazon, B.; Rodriguez-Sanchez, A.; Hurtado-Martinez, M.; Gonzalez-Lopez, J.; Pfetzing, P.; Gonzalez-Martinez, A. Performance and Microbial Community Structure of Aerobic Granular Bioreactors at Different Operational Temperature. J. Water Process Eng. 2020, 33, 101110. [Google Scholar] [CrossRef]
- Xu, D.; Liu, J.; Ma, T.; Gao, Y.; Zhang, S.; Li, J. Rapid Granulation of Aerobic Sludge in a Continuous-Flow Reactor with a Two-Zone Sedimentation Tank by the Addition of Dewatered Sludge. J. Water Process Eng. 2021, 41, 101941. [Google Scholar] [CrossRef]
- Sun, Y.; Angelotti, B.; Wang, Z.W. Continuous-Flow Aerobic Granulation in Plug-Flow Bioreactors Fed with Real Domestic Wastewater. Sci. Total Environ. 2019, 688, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Bin, L.; Tang, B.; Huang, S.; Fu, F.; Chen, Q.; Wu, L.; Wu, C. Cultivating Granular Sludge Directly in a Continuous-Flow Membrane Bioreactor with Internal Circulation. Chem. Eng. J. 2017, 309, 108–117. [Google Scholar] [CrossRef]
- Bumbac, C.; Ionescu, I.A.; Tiron, O.; Badescu, V.R. Continuous Flow Aerobic Granular Sludge Reactor for Dairy Wastewater Treatment. Water Sci. Technol. 2015, 71, 440–445. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burtun, F.L.; Stensel, H.D. Wastewater Engineering Treatment and Reuse, 4th ed.; Mic Graw-Hill: New York, NY, USA, 2003; ISBN 0070418780. [Google Scholar]
- Tocchi, C.; Federici, E.; Fidati, L.; Manzi, R.; Vincigurerra, V.; Petruccioli, M. Aerobic Treatment of Dairy Wastewater in an Industrial Three-Reactor Plant: Effect of Aeration Regime on Performances and on Protozoan and Bacterial Communities. Water Res. 2012, 46, 3334–3344. [Google Scholar] [CrossRef] [PubMed]
- Baird, R.B.; Eaton, A.D.; Rice, E.W. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation, Eds.; APHA: Washington, DC, USA, 2017; ISBN 087553287X. [Google Scholar]
- Tauber, J.; Flesch, B.; Parravicini, V.; Svardal, K.; Krampe, J. Influence of Road Salt Thawing Peaks on the Inflow Composition and Activated Sludge Properties in Municipal Wastewater Treatment. Water Sci. Technol. 2021, 84, 314–322. [Google Scholar] [CrossRef]
- Hultman, B.; Lowen, M.; Karlsson, U.; Li, P.H.; Molina, L. Prediction of Activated Sludge Sedimentation Based on Sludge Indices. Water Sci. Technol. 1991, 24, 33–42. [Google Scholar] [CrossRef]
- Ntamitrou, M.D.; Papakonstantinou, A.; Sklari, S.; Samaras, P. Evaluation of the Operation Performance of a Municipal Activated Sludge Unit. Desalin. Water Treat. 2012, 39, 271–277. [Google Scholar] [CrossRef]
- Arregui, L.; Liébana, R.; Calvo, P.; Pérez-Uz, B.; Salvadó, H.; Serrano, S. Bioindication in Activated Sludge Wastewater Treatment Plants. In Handbook of Wastewater Treatment: Biological Methods, Technology and Environmental Impact; Nova Science Publishers: Hauppauge, NY, USA, 2013; pp. 277–291. [Google Scholar]
- Li, Z.H.; Kuba, T.; Kusuda, T. Aerobic Granular Sludge: A Promising Technology for Decentralised Wastewater Treatment. Water Sci. Technol. 2006, 53, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Othman, I.; Anuar, A.N.; Ujang, Z.; Rosman, N.H.; Harun, H.; Chelliapan, S. Livestock Wastewater Treatment Using Aerobic Granular Sludge. Bioresour. Technol. 2013, 133, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, Z.; Shen, J.; Wang, X. Performance of Aerobic Granular Sludge in Different Bioreactors. Environ. Technol. 2014, 35, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.A.; Guimarães, L.B.; Magnus, B.S.; Leite, W.R.; Vilar, V.J.P.; da Costa, R.H.R. How Volumetric Exchange Ratio and Carbon Availability Contribute to Enhance Granular Sludge Stability in a Fill/Draw Mode SBR Treating Domestic Wastewater? J. Water Process Eng. 2021, 40, 101917. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, L.; Lu, W.; Li, Y.; Liu, P.; Nie, K. Cultivation of Aerobic Granular Sludge in a Conventional, Continuous Flow, Completely Mixed Activated Sludge System. Front. Environ. Sci. Eng. 2015, 9, 324–333. [Google Scholar] [CrossRef]
- Deng, S.; Wang, L.; Su, H. Role and Influence of Extracellular Polymeric Substances on the Preparation of Aerobic Granular Sludge. J. Environ. Manag. 2016, 173, 49–54. [Google Scholar] [CrossRef]
- Sajjad, M.; Kim, I.S.; Kim, K.S. Development of a Novel Process to Mitigate Membrane Fouling in a Continuous Sludge System by Seeding Aerobic Granules at Pilot Plant. J. Memb. Sci. 2016, 497, 90–98. [Google Scholar] [CrossRef]
- Fiałkowska, E.; Pajdak-Stós, A. The Role of Lecane Rotifers in Activated Sludge Bulking Control. Water Res. 2008, 42, 2483–2490. [Google Scholar] [CrossRef]
- Kocerba-Soroka, W.; Fiałkowska, E.; Pajdak-Stós, A.; Sobczyk, M.; Pławecka, M.; Fyda, J. Effect of the Rotifer Lecane Inermis, a Potential Sludge Bulking Control Agent, on Process Parameters in a Laboratory-Scale SBR System. Water Sci. Technol. 2013, 68, 2012–2018. [Google Scholar] [CrossRef]
- Morales, N.; Figueroa, M.; Campos, J.L.; Méndez, R. Aerobic Granular-Type Biomass Development in a Continuous Stirred Tank Reactor. Sep. Purif. Technol. 2012, 89, 199–205. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, W.; Liang, D.T.; Tay, J.H. Structure and Stability of Aerobic Granules Cultivated under Different Shear Force in Sequencing Batch Reactors. Appl. Microbiol. Biotechnol. 2007, 76, 1199–1208. [Google Scholar] [CrossRef]
- Val Del Río, A.; Figueroa, M.; Mosquera-Corral, A.; Campos, J.L.; Méndez, R. Stability of Aerobic Granular Biomass Treating the Effluent from a Seafood Industry. Int. J. Environ. Res. 2013, 7, 265–276. [Google Scholar] [CrossRef]
- Jungles, M.K.; Campos, J.L.; Costa, R.H.R. Sequencing Batch Reactor Operation for Treating Wastewater with Aerobic Granular Sludge. Braz. J. Chem. Eng. 2014, 31, 27–33. [Google Scholar] [CrossRef] [Green Version]
- khan, M.Z.; Mondal, P.K.; Sabir, S. Bioremediation of 2-Chlorophenol Containing Wastewater by Aerobic Granules-Kinetics and Toxicity. J. Hazard. Mater. 2011, 190, 222–228. [Google Scholar] [CrossRef]
- Yu, C.; Wang, K.; Tian, C.; Yuan, Q. Aerobic Granular Sludge Treating Low-Strength Municipal Wastewater: Efficient Carbon, Nitrogen and Phosphorus Removal with Hydrolysis-Acidification Pretreatment. Sci. Total Environ. 2021, 792, 148297. [Google Scholar] [CrossRef] [PubMed]
- Henriet, O.; Meunier, C.; Henry, P.; Mahillon, J. Improving Phosphorus Removal in Aerobic Granular Sludge Processes through Selective Microbial Management. Bioresour. Technol. 2016, 211, 298–306. [Google Scholar] [CrossRef]
- Ji, G.; Zhai, F.; Wang, R.; Ni, J. Sludge Granulation and Performance of a Low Superficial Gas Velocity Sequencing Batch Reactor (SBR) in the Treatment of Prepared Sanitary Wastewater. Bioresour. Technol. 2010, 101, 9058–9064. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.F.; Liu, Q.S.; Tay, J.H. The Role of Cell Hydrophobicity in the Formation of Aerobic Granules. Curr. Microbiol. 2003, 46, 270–274. [Google Scholar] [CrossRef]
- Rosman, N.H.; Nor Anuar, A.; Chelliapan, S.; Md Din, M.F.; Ujang, Z. Characteristics and Performance of Aerobic Granular Sludge Treating Rubber Wastewater at Different Hydraulic Retention Time. Bioresour. Technol. 2014, 161, 155–161. [Google Scholar] [CrossRef]
- López-Palau, S.; Dosta, J.; Mata-Álvarez, J. Start-up of an Aerobic Granular Sequencing Batch Reactor for the Treatment of Winery Wastewater. Water Sci. Technol. 2009, 60, 1049–1054. [Google Scholar] [CrossRef]
- Liu, Y.; Tay, J. Influence of Starvation Time on Formation and Stability of Aerobic Granules in Sequencing Batch Reactors. Bioresour. Technol. 2008, 99, 980–985. [Google Scholar] [CrossRef]
- Liu, Y.; Tay, J. Variable Aeration in Sequencing Batch Reactor with Aerobic Granular Sludge. J. Biotechnol. 2006, 124, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Angelotti, B.; Brooks, M.; Wang, Z.W. Feast/Famine Ratio Determined Continuous Flow Aerobic Granulation. Sci. Total Environ. 2021, 750, 141467. [Google Scholar] [CrossRef]
- Ministério do Ambiente Decreto-Lei n.o 236/98. Diário da República n.o 176/1998, Série I-A 1998-08-01 1998, No 176, 3676–3722.
- Zhu, T.; Zhang, Y.; Quan, X.; Li, H. Effects of an Electric Field and Iron Electrode on Anaerobic Denitrification at Low C/N Ratios. Chem. Eng. J. 2015, 266, 241–248. [Google Scholar] [CrossRef]
- Chai, H.; Xiang, Y.; Chen, R.; Shao, Z.; Gu, L.; Li, L.; He, Q. Enhanced Simultaneous Nitrification and Denitrification in Treating Low Carbon-to-Nitrogen Ratio Wastewater: Treatment Performance and Nitrogen Removal Pathway. Bioresour. Technol. 2019, 280, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Torregrossa, M. Simultaneous Nitrogen and Organic Carbon Removal in Aerobic Granular Sludge Reactors Operated with High Dissolved Oxygen Concentration. Bioresour. Technol. 2013, 142, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wei, Z.; Li, S.; Lao, H.; Wang, W.; Zeng, H. Chemosphere Performance and Operational Strategy of Simultaneous Nitri Fi Cation, Denitri Fi Cation, and Phosphorus Removal System under the Condition of Low Organic Loading Rate in Wet Weather. Chemosphere 2021, 270, 129464. [Google Scholar] [CrossRef]
- Kang, A.J.; Yuan, Q. Long-Term Stability and Nutrient Removal Efficiency of Aerobic Granules at Low Organic Loads. Bioresour. Technol. 2017, 234, 336–342. [Google Scholar] [CrossRef]
- Tay, J.-H.; Pan, S.; He, Y.; Tay, S.T.L. Effect of Organic Loading Rate on Aerobic Granulation. II: Characteristics of Aerobic Granules. J. Environ. Eng. 2004, 130, 1102–1109. [Google Scholar] [CrossRef]
- Lochmatter, S.; Gonzalez-Gil, G.; Holliger, C. Optimized Aeration Strategies for Nitrogen and Phosphorus Removal with Aerobic Granular Sludge. Water Res. 2013, 47, 6187–6197. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Huang, W.; Li, H.; Lei, Z.; Zhang, Z.; Tay, J.H.; Lee, D.J. Species and Distribution of Inorganic and Organic Phosphorus in Enhanced Phosphorus Removal Aerobic Granular Sludge. Bioresour. Technol. 2015, 193, 549–552. [Google Scholar] [CrossRef]
- Wang, H.; Song, Q.; Wang, J.; Zhang, H.; He, Q.; Zhang, W.; Song, J.; Zhou, J.; Li, H. Simultaneous Nitrification, Denitrification and Phosphorus Removal in an Aerobic Granular Sludge Sequencing Batch Reactor with High Dissolved Oxygen: Effects of Carbon to Nitrogen Ratios. Sci. Total Environ. 2018, 642, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Song, K.G.; Cho, J.; Ahn, K.H. Effects of Internal Recycling Time Mode and Hydraulic Retention Time on Biological Nitrogen and Phosphorus Removal in a Sequencing Anoxic/Anaerobic Membrane Bioreactor Process. Bioprocess Biosyst. Eng. 2009, 32, 135–142. [Google Scholar] [CrossRef]
- Nicholls, H.A. Kinetics of phosphorus transformations in aerobic and anaerobic environments. In Kinetics of Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 1979; pp. 89–102. [Google Scholar]
- Lötter, L.H.; Murphy, M. Microscopic Evaluation of Carbon and Phosphorus Accumulation in Nutrient Removal Activated Sludge Plants. Water Sci. Technol. 1988, 20, 37–49. [Google Scholar] [CrossRef]
- Sarma, S.J.; Tay, J.H. Carbon, Nitrogen and Phosphorus Removal Mechanisms of Aerobic Granules. Crit. Rev. Biotechnol. 2018, 38, 1077–1088. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Wang, Q.; Yuan, T.; Lei, Z.; Zhang, Z.; Shimizu, K.; Lee, D.J. Simultaneous Recovery of Phosphorus and Alginate-like Exopolysaccharides from Two Types of Aerobic Granular Sludge. Bioresour. Technol. 2022, 346, 126411. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Wang, Q.; Lei, Z.; Yuan, T.; Shimizu, K.; Zhang, Z.; Adachi, Y.; Lee, D.J.; Chen, R. Achieving Stably Enhanced Biological Phosphorus Removal from Aerobic Granular Sludge System via Phosphorus Rich Liquid Extraction during Anaerobic Period. Bioresour. Technol. 2022, 346, 126439. [Google Scholar] [CrossRef]
- de Carvalho, C.D.A.; dos Santos, A.F.; Tavares Ferreira, T.J.; Sousa Aguiar Lira, V.N.; Mendes Barros, A.R.; dos Santos, A.B. Resource Recovery in Aerobic Granular Sludge Systems: Is It Feasible or Still a Long Way to Go? Chemosphere 2021, 274, 129881. [Google Scholar] [CrossRef]





| Setup | Characteristics | Results | Ref |
|---|---|---|---|
| Column-type (120 × 6 cm) |
|
| [41] |
| Airlift bioreactor with a settling tank and a membrane |
|
| [49] |
| Double column cyclic reactor (188 × 8.4 cm) |
|
| [42] |
| 5 CSTRs in serie |
|
| [50] |
| Reverse flow baffled reactor |
|
| [51] |
| Continuous flow airlift fluidized bed reactor |
|
| [52] |
| Aerobic upflow fluidized bed |
|
| [53] |
| Anaerobic and aerobic reactors, and a settling tank |
|
| [54] |
| Continuous flow membrane bioreactor |
|
| [55] |
| COD (mg/L) | sCOD (mg/L) | TC (mg/L) | TN (mg/L) | TP (mg/L) | pH | Ref. |
|---|---|---|---|---|---|---|
| 1031.3 ± 50.1 | 839 ± 30 | 516.9 ± 2.9 | 109.6 ± 13.5 | 7.14 ± 0.41 | 6.03 ± 1.48 | Used in this study |
| 662–1293 | - | - | 8.1–38.8 | 0.79–6.84 * | 5.3–7.0 | [62] |
| Phase | Operating Time (Days) | Feeding Flow (L/d) | Recycle Flow (L/d) | OLR (kg COD/(m3·d)) | Total HRT (d) |
|---|---|---|---|---|---|
| 1 | 23 | 5.3 | 7.2 | 0.234 | 8.01 |
| 2 | 27 | 10.1 | 7.2 | 0.445 | 4.20 |
| 3 | 25 | 15.1 | 20.2 | 0.666 | 2.81 |
| 4 | 15 | 15.1 | 36.2 | 0.666 | 2.81 |
| TP (mg/L) | |||
|---|---|---|---|
| Phase | Anaerobic Reactor-1 | Aerobic Reactor-2 | Effluent |
| 1 | 0.53 | 0.53 | 1.15 |
| 2 | 2.84 | 1.34 | 1.34 |
| 3 | 2.38 | 3.72 | 3.16 |
| 4 | 3.58 | 1.23 | 1.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.F.; Silva, J.R.; Santos, A.D.; Vicente, C.; Dries, J.; Castro, L.M. Continuous-Flow Aerobic Granular Sludge Treatment of Dairy Wastewater. Water 2023, 15, 1066. https://doi.org/10.3390/w15061066
Silva JF, Silva JR, Santos AD, Vicente C, Dries J, Castro LM. Continuous-Flow Aerobic Granular Sludge Treatment of Dairy Wastewater. Water. 2023; 15(6):1066. https://doi.org/10.3390/w15061066
Chicago/Turabian StyleSilva, João F., João R. Silva, Andreia D. Santos, Carolina Vicente, Jan Dries, and Luis M. Castro. 2023. "Continuous-Flow Aerobic Granular Sludge Treatment of Dairy Wastewater" Water 15, no. 6: 1066. https://doi.org/10.3390/w15061066