Microbial and Biogeochemical Shifts in a Highly Anthropogenically Impacted Estuary (“El Sauce” Valparaíso)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Procedures
2.2. Laboratory Procedures for Biochemical Parameters
2.3. Sediment Granulometry, Organic Matter, and Isotopic Analyses
2.4. Benthic Microbial Community Characterization Using Molecular Approaches
2.5. Quantitative PCR Analyses
2.6. Sequencing Data Curation and Taxonomic Classification Processing
2.7. Statistical Analyses
3. Results
3.1. Environmental Factors, Nutrients, and Water Quality Variables
3.2. GHG Distribution along El Sauce Estuary
3.3. Sedimentary Conditions and Potential Organic Matter Quality
3.4. Spearman Correlation Analyses Associated with Water Quality Parameters and GHG
3.5. Benthic Microbial Community Composition
3.6. Functional Microbial Groups Spatial Variability during Winter and Summer in El Sauce Estuary
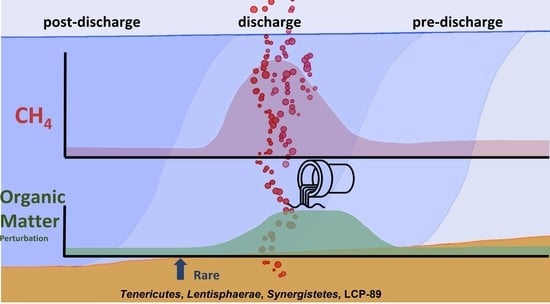
4. Discussion
4.1. The WWTP Altered Physicochemical Properties of El Sauce Estuary
4.2. Benthic Microbial Communities and Functional Groups of El Sauce Estuary
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bianchi, T.S. Biogeochemistry of Estuaries; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Vincent, S.G.T.; Reshmi, R.; Hassan, S.J.; Nair, K.D.; Varma, A. Predominant terminal electron accepting processes during organic matter degradation: Spatio-temporal changes in Ashtamudi estuary, Kerala, India. Estuar. Coast. Shelf Sci. 2017, 198, 508–517. [Google Scholar] [CrossRef]
- Kirwan, M.L.; Megonigal, J.P. Tidal wetland stability in the face of human impacts and sea-level rise. Nature 2013, 504, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Wu, W.; Yang, Z.; Zhou, Y. Drivers, trends, and potential impacts of long-term coastal reclamation in China from 1985 to 2010. Estuar. Coast. Shelf Sci. 2016, 170, 83–90. [Google Scholar] [CrossRef]
- Chen, X.; Zong, Y.; Zhang, E.; Xu, J.; Li, S. Human impacts on the Changjiang (Yangtze) River basin, China, with special reference to the impacts on the dry season water discharges into the sea. Geomorphology 2001, 41, 111–123. [Google Scholar] [CrossRef]
- Haarstad, K.; Bavor, H.J.; Maehlum, T. Organic and metallic pollutants in water treatment and natural wetlands: A review. Water Sci. Technol. 2012, 65, 76–99. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.A.; Savage, K.E.; Bettez, N.; Marino, R.; Howarth, R. Nitrogen in Runoff from Residential Roads in a Coastal Area. Water Air Soil Pollut. 2009, 210, 3–13. [Google Scholar] [CrossRef]
- Siddiqui, Z. Holistic Approach to Mitigate the Pollution Impacts in the Coastal Ecosystem of Thailand Using the Remote Sensing Techniques. Int. J. Environ. Res. 2011, 5, 297–306. [Google Scholar]
- Findlay, S.; Fischer, D. Ecosystem attributes related to tidal wetland effects on water quality. Ecology 2013, 94, 117–125. [Google Scholar] [CrossRef]
- Deegan, L.A.; Johnson, D.S.; Warren, R.S.; Peterson, B.J.; Fleeger, J.W.; Fagherazzi, S.; Wollheim, W.M. Coastal eutrophication as a driver of salt marsh loss. Nature 2012, 490, 388–392. [Google Scholar] [CrossRef]
- Savage, C.; Thrush, S.F.; Lohrer, A.M.; Hewitt, J.E. Ecosystem Services Transcend Boundaries: Estuaries Provide Resource Subsidies and Influence Functional Diversity in Coastal Benthic Communities. PLoS ONE 2012, 7, e42708. [Google Scholar] [CrossRef]
- Harvey, H.; Dyda, R.; Kirchman, D. Impact of DOM composition on bacterial lipids and community structure in estuaries. Aquat. Microb. Ecol. 2006, 42, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Osterholz, H.; Kirchman, D.L.; Niggemann, J.; Dittmar, T. Diversity of bacterial communities and dissolved organic matter in a temperate estuary. FEMS Microbiol. Ecol. 2018, 94, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Traving, S.J.; Rowe, O.; Jakobsen, N.M.; Sørensen, H.; Dinasquet, J.; Stedmon, C.A.; Andersson, A.; Riemann, L. The Effect of Increased Loads of Dissolved Organic Matter on Estuarine Microbial Community Composition and Function. Front. Microbiol. 2017, 8, 351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Wang, L.; Zhang, X.; He, M.; Wang, D.; Ren, Y.; Yao, H.; Ngegla, J.N.V.; Pan, H. Effects of different types of anthropogenic disturbances and natural wetlands on water quality and microbial communities in a typical black-odor river. Ecol. Indic. 2022, 136, 108613. [Google Scholar] [CrossRef]
- Golovko, O.; Örn, S.; Sörengård, M.; Frieberg, K.; Nassazzi, W.; Lai, F.Y.; Ahrens, L. Occurrence and removal of chemicals of emerging concern in wastewater treatment plants and their impact on receiving water systems. Sci. Total. Environ. 2020, 754, 142122. [Google Scholar] [CrossRef]
- Sörengård, M.; Campos-Pereira, H.; Ullberg, M.; Lai, F.Y.; Golovko, O.; Ahrens, L. Mass loads, source apportionment, and risk estimation of organic micropollutants from hospital and municipal wastewater in recipient catchments. Chemosphere 2019, 234, 931–941. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Gros, M.; Ahrens, L.; Wiberg, K. Impact of on-site, small and large scale wastewater treatment facilities on levels and fate of pharma-ceuticals, personal care products, artificial sweeteners, pesticides, and perfluoroalkyl substances in recipient waters. Sci. Total Environ. 2017, 601, 1289–1297. [Google Scholar] [CrossRef]
- Lawrie, R.A.; Stretch, D.D.; Perissinotto, R. The effects of wastewater discharges on the functioning of a small temporarily open/closed estuary. Estuar. Coast. Shelf Sci. 2010, 87, 237–245. [Google Scholar] [CrossRef]
- Santos, I.R.; Costa, R.C.; Freitas, U.; Fillmann, G. Influence of effluents from a Wastewater Treatment Plant on nutrient distribution in a coastal creek from southern Brazil. Braz. Arch. Biol. Technol. 2008, 51, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, M.; Abad, D.; Albaina, A.; Cralle, L.; Goñi-Urriza, M.S.; Estonba, A.; Zarraonaindia, I. Unraveling the environmental and anthropogenic drivers of bacterial community changes in the Estuary of Bilbao and its tributaries. PLoS ONE 2017, 12, e0178755. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, V.F.; Reis-Santos, P.; Duarte, B.; Cabral, H.N.; Caçador, M.I.; Vaz, N.; Dias, J.M.; Pais, M.P. Roving pharmacies: Modelling the dispersion of pharmaceutical contamination in estuaries. Ecol. Indic. 2020, 115, 106437. [Google Scholar] [CrossRef]
- Shao, S.; Tai, X.; Zhen, F.; Li, J.; Li, Y. Organic Carbon in Urban Wetland Sediments and Implication for Potential Greenhouse Gas Emission. In Euro-Mediterranean Conference for Environmental Integration; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Amaral, V.; Ortega, T.; Romera-Castillo, C.; Forja, J. Linkages between greenhouse gases (CO2, CH4, and N2O) and dissolved organic matter composition in a shallow estuary. Sci. Total. Environ. 2021, 788, 147863. [Google Scholar] [CrossRef] [PubMed]
- Euler, S.; Jeffrey, L.C.; Maher, D.T.; MacKenzie, D.; Tait, D.R. Shifts in methanogenic archaea communities and methane dynamics along a subtropical estuarine land use gradient. PLoS ONE 2020, 15, e0242339. [Google Scholar] [CrossRef] [PubMed]
- Vincent, S.; Salahudeen, J.; Godson, P.; Abhijith, S.; Nath, A.; Krishnan, K.; Magesh, N.; Kumar, S.; Moses, S. Environmental factors influencing methanogenic activity in two contrasting tropical lake sediments. J. Environ. Biol. 2021, 42, 211–219. [Google Scholar] [CrossRef]
- Burdon, F.J.; Bai, Y.; Reyes, M.; Tamminen, M.; Staudacher, P.; Mangold, S.; Singer, H.; Räsänen, K.; Joss, A.; Tiegs, S.D.; et al. Stream microbial communities and ecosystem functioning show complex responses to multiple stressors in wastewater. Glob. Change Biol. 2020, 26, 6363–6382. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Cai, P.; Gao, Q.; Zhong, H.; Sun, W.; Chen, Q. Impacts of municipal wastewater treatment plant discharge on microbial community structure and function of the receiving river in Northwest Tibetan Plateau. J. Hazard. Mater. 2021, 423, 127170. [Google Scholar] [CrossRef]
- Guo, X.-P.; Yang, Y.; Niu, Z.-S.; Lu, D.-P.; Zhu, C.-H.; Feng, J.-N.; Wu, J.-Y.; Chen, Y.-R.; Tou, F.-Y.; Liu, M.; et al. Characteristics of microbial community indicate anthropogenic impact on the sediments along the Yangtze Estuary and its coastal area, China. Sci. Total Environ. 2018, 648, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Mansfeldt, C.; Deiner, K.; Mächler, E.; Fenner, K.; Eggen, R.I.; Stamm, C.; Schönenberger, U.; Walser, J.-C.; Altermatt, F. Microbial community shifts in streams receiving treated wastewater effluent. Sci. Total. Environ. 2019, 709, 135727. [Google Scholar] [CrossRef]
- Rivera Castro, C.A.; Pino, J.A.L.; Pizarro, B.A.; del Pilar Tobar Correa, T.; Lepe, C.L.T.; Figueroa, A.M.C.; Geisse, A.R.; Castro, M.Á.R. Water quality in the El Sauce Estuary, Valparaíso, central Chile. Rev. Int. Contam. Ambient 2020, 36, 261–273. [Google Scholar]
- División de Estudios y Planificación STD N° 364. Inventario de Cuencas, Subcuencas y Subsubcuencas de Chile; Dirección General de Aguas: Santiago, Chile, 2014; p. 16. [Google Scholar]
- Laura Bridgewater; American Public Health Association; American Water Works Association; Water Environment Federation. Book Standard Methods for the Examination of Water and Wastewater; Mc Graw Hill: New York, NY, USA, 2005. [Google Scholar]
- Gray, M.; Pratte, Z.; Kellogg, C. Comparison of DNA preservation methods for environmental bacterial community samples. FEMS Microbiol. Ecol. 2013, 83, 468–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strickland, J.D.H.; Parsons, T. A Practical Handbook of Seawater Analysis; Fisheries Research Board of Canada: Ottawa, ON, Canada, 1972. [Google Scholar]
- Atlas, E.L.; Hager, S.; Gordon, L.; Park, P. A Practical Manual for Use of the Technicon (Trade Name) Autoanalyzer (Trade Name) in Seawater Nutrient Analyses; Revised; Oregon State Univ Corvallis Dept Of Oceanography: Corvallis, OR, USA, 1971. [Google Scholar]
- Holm-Hansen, O.; Lorenzen, C.J.; Holmes, R.W.; Strickland, J.D.H. Fluorometric Determination of Chlorophyll. ICES J. Mar. Sci. 1965, 30, 3–15. [Google Scholar] [CrossRef]
- McAuliffe, C. Gas chromatographic determination of solutes by multiple phase equilibrium. Chem. Technol. 1971, 1, 46–51. [Google Scholar]
- Friedman, G.M.; Sanders, J. Principles of Sedimentology; Wiley: Hoboken, NJ, USA, 1978. [Google Scholar]
- Byers, S.C.; Mills, E.L.; Stewart, P.L. A comparison of methods of determining organic carbon in marine sediments, with suggestions for a standard method. Hydrobiologia 1978, 58, 43–47. [Google Scholar] [CrossRef]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Apprill, A.; McNally, S.; Parsons, R.; Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterio-plankton. Aquat. Microb. Ecol. 2015, 75, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pjevac, P.; Schauberger, C.; Poghosyan, L.; Herbold, C.; van Kessel, M.; Daebeler, A.; Steinberger, M.; Jetten, M.; Lücker, S.; Wagner, M.; et al. AmoA-targeted polymerase chain reaction primers for the specific detection and quantification of comammox Nitrospira in the environment. Front. Microbiol. 2017, 8, 1508. [Google Scholar] [CrossRef] [Green Version]
- Holmes, A.J.; Costello, A.; Lidstrom, M.E.; Murrell, J.C. Evidence that participate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol. Lett. 1995, 132, 203–208. [Google Scholar] [CrossRef]
- Costello, A.M.; Lidstrom, M.E. Molecular Characterization of Functional and Phylogenetic Genes from Natural Populations of Methanotrophs in Lake Sediments. Appl. Environ. Microbiol. 1999, 65, 5066–5074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinberg, L.M.; Regan, J.M. Phylogenetic Comparison of the Methanogenic Communities from an Acidic, Oligotrophic Fen and an Anaerobic Digester Treating Municipal Wastewater Sludge. Appl. Environ. Microbiol. 2008, 74, 6663–6671. [Google Scholar] [CrossRef] [Green Version]
- Molina, V.; Belmar, L.; Levipan, H.A.; Ramírez-Flandes, S.; Anguita, C.; Galán, A.; Montes, I.; Ulloa, O. Spatiotemporal Distribution of Key Pelagic Microbes in a Seasonal Oxygen-Deficient Coastal Upwelling System of the Eastern South Pacific Ocean. Front. Mar. Sci. 2020, 7, 561597. [Google Scholar] [CrossRef]
- Cabrol, L.; Thalasso, F.; Gandois, L.; Sepulveda-Jauregui, A.; Martinez-Cruz, K.; Teisserenc, R.; Tananaev, N.; Tveit, A.; Svenning, M.M.; Barret, M. Anaerobic oxidation of methane and associated microbiome in anoxic water of Northwestern Siberian lakes. Sci. Total. Environ. 2020, 736, 139588. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.; Bokulich, N.; Abnet, C.; Al-Ghalith, G.; Alexander, H.; Alm, E.; Arumugam, M.; Asnicar, F.; et al. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 2016, e2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Team, R.C. R: A Language and Environmental for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Harrell; Dupont, C. Harrell Miscellaneous, R Package, Version 4.6-0. 2021. Available online: https://cran.r-project.org/package=Hmisc (accessed on 1 November 2022).
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package “corrplot”: Visualization of a Correlation Matrix. 2017. Available online: https://github.com/taiyun/corrplot (accessed on 1 November 2022).
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, K.S.; Kirkegaard, R.H.; Karst, S.M.; Albertsen, M. Ampvis2: An R package to analyse and visualise 16S rRNA amplicon data. BioRxiv 2018, 299537. [Google Scholar]
- Oksanen, J.; Guillaume, B.; Kindt, R.; Pierre, L. Community Ecology Package, R Package Version. 2013; Volume 2, pp. 321–326. Available online: cran.r-project.org/package=vegan (accessed on 1 November 2022).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.; Gorley, R.; Clarke, R. Permanova+ for Primer: Guide to Software and Statisticl Methods; Primer-E Limited: Auckland, New Zealand, 2008. [Google Scholar]
- Hu, B.; Wang, D.; Zhou, J.; Meng, W.; Li, C.; Sun, Z.; Guo, X.; Wang, Z. Greenhouse gases emission from the sewage draining rivers. Sci. Total. Environ. 2018, 612, 1454–1462. [Google Scholar] [CrossRef]
- Burgos, M.; Sierra, A.; Ortega, T.; Forja, J. Anthropogenic effects on greenhouse gas (CH4 and N2O) emissions in the Guadalete River Estuary (SW Spain). Sci. Total. Environ. 2015, 503–504, 179–189. [Google Scholar] [CrossRef]
- Ivanov, M.; Pimenov, N.; Rusanov, I.; Lein, A. Microbial Processes of the Methane Cycle at the North-western Shelf of the Black Sea. Estuar. Coast. Shelf Sci. 2002, 54, 589–599. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; Shuster, W.D.; Rebholz, J.A. Nitrous Oxide Emissions from a Large, Impounded River: The Ohio River. Environ. Sci. Technol. 2010, 44, 7527–7533. [Google Scholar] [CrossRef]
- Hinshaw, S.E.; Dahlgren, R.A. Dissolved Nitrous Oxide Concentrations and Fluxes from the Eutrophic San Joaquin River, California. Environ. Sci. Technol. 2013, 47, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Sarma, V.V.S.S.; Arya, J.; Subbaiah, C.V.; Naidu, S.A.; Gawade, L.; Kumar, P.P.; Reddy, N.P.C. Stable isotopes of carbon and nitrogen in suspended matter and sediments from the Godavari estuary. J. Oceanogr. 2012, 68, 307–319. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Herman, P.M.J. Organic matter processing in tidal estuaries. Mar. Chem. 2007, 106, 127–147. [Google Scholar] [CrossRef]
- Vaalgamaa, S.; Sonninen, E.; Korhola, A.; Weckström, K. Identifying recent sources of organic matter enrichment and eutrophication trends at coastal sites using stable nitrogen and carbon isotope ratios in sediment cores. J. Paleolimnol. 2013, 50, 191–206. [Google Scholar] [CrossRef]
- Voss, M.; Emeis, K.-C.; Hille, S.; Neumann, T.; Dippner, J.W. Nitrogen cycle of the Baltic Sea from an isotopic perspective. Glob. Biogeochem. Cycles 2005, 19. [Google Scholar] [CrossRef] [Green Version]
- Pozo-Solar, F.; Cornejo-D´ottone, M.; Orellana, R.; Yepsen, D.V.; Bassi, N.; Salcedo-Castro, J.; Aguilar-Muñoz, P.; Molina, V. Dissolved greenhouse gases and benthic microbial communities in coastal wetlands of the Chilean coast semiarid region. PLoS ONE 2022, 17, e0271208. [Google Scholar] [CrossRef]
- Wyness, A.J.; Fortune, I.; Blight, A.J.; Browne, P.; Hartley, M.; Holden, M.; Paterson, D.M. Ecosystem engineers drive differing microbial community composition in intertidal estuarine sediments. PLoS ONE 2021, 16, e0240952. [Google Scholar] [CrossRef]
- Niestępski, S.; Harnisz, M.; Ciesielski, S.; Korzeniewska, E.; Osińska, A. Environmental fate of Bacteroidetes, with particular emphasis on Bacteroides fragilis group bacteria and their specific antibiotic resistance genes, in activated sludge wastewater treatment plants. J. Hazard. Mater. 2020, 394, 122544. [Google Scholar] [CrossRef]
- Price, J.R.; Ledford, S.H.; Ryan, M.O.; Toran, L.; Sales, C.M. Wastewater treatment plant effluent introduces recoverable shifts in microbial community composition in receiving streams. Sci. Total. Environ. 2018, 613–614, 1104–1116. [Google Scholar] [CrossRef]
- McLellan, S.L.; Huse, S.M.; Mueller-Spitz, S.R.; Andreishcheva, E.N.; Sogin, M.L. Diversity and population structure of sewage-derived microorganisms in wastewater treatment plant influent. Environ. Microbiol. 2010, 12, 378–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Ju, F.; Zhang, T. Tracking human sewage microbiome in a municipal wastewater treatment plant. Appl. Microbiol. Biotechnol. 2013, 98, 3317–3326. [Google Scholar] [CrossRef]
- Drury, B.; Rosi-Marshall, E.; Kelly, J.J. Wastewater Treatment Effluent Reduces the Abundance and Diversity of Benthic Bacterial Communities in Urban and Suburban Rivers. Appl. Environ. Microbiol. 2013, 79, 1897–1905. [Google Scholar] [CrossRef] [Green Version]
- Tertuliani, J.; Alvarez, D.; Furlong, E.; Meyer, M.; Zaugg, S.; Koltun, G. Occurrence of Organic Wastewater Compounds in the Tinkers Creek Watershed and Two Other Tributaries to the Cuyahoga River, Northeast Ohio; U.S. Geological Survey: Reston, VA, USA, 2008.
- Zerva, I.; Remmas, N.; Kagalou, I.; Melidis, P.; Ariantsi, M.; Sylaios, G.; Ntougias, S. Effect of Chlorination on Microbiological Quality of Effluent of a Full-Scale Wastewater Treatment Plant. Life 2021, 11, 68. [Google Scholar] [CrossRef]
- Dang, H.; Li, J.; Chen, R.; Wang, L.; Guo, L.; Zhang, Z.; Klotz, M. Diversity, abundance, and spatial distribution of sediment ammonia-oxidizing Betaproteobacteria in response to environmental gradients and coastal eutrophication in Jiaozhou Bay, China. Appl. Environ. Microbiol. 2010, 76, 4691–4702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, L.; Sun, R.; Dai, T.; Tian, J.; Wen, D. Ammonia-oxidizing bacteria and archaea in wastewater treatment plant sludge and nearby coastal sediment in an industrial area in China. Appl. Microbiol. Biotechnol. 2015, 99, 4495–4507. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, G.; Hua, Y.; Wan, X.; Hu, J.; Zhu, D.; Zhao, J. The diversity of comammox bacteria and the effect of sewage discharge on their abundance in eutrophic lake sediments. J. Soils Sediments 2020, 20, 2495–2503. [Google Scholar] [CrossRef]
- Hu, B.-L.; Shen, L.-D.; Lian, X.; Zhu, Q.; Liu, S.; Huang, Q.; He, Z.-F.; Geng, S.; Cheng, D.-Q.; Lou, L.-P.; et al. Evidence for nitrite-dependent anaerobic methane oxidation as a previously overlooked microbial methane sink in wetlands. Proc. Natl. Acad. Sci. USA 2014, 111, 4495–4500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naqvi, S.W.A.; Lam, P.; Narvenkar, G.; Sarkar, A.; Naik, H.; Pratihary, A.; Shenoy, D.M.; Gauns, M.; Kurian, S.; Damare, S.; et al. Methane stimulates massive nitrogen loss from freshwater reservoirs in India. Nat. Commun. 2018, 9, 1265. [Google Scholar] [CrossRef] [PubMed]
- Deb, D.; Schneider, P.; Dudayev, Z.; Emon, A.; Areng, S.S.; Mozumder, M.M. Perceptions of urban pollution of river dependent rural communities and their impact: A case study in Bang-ladesh. Sustainability 2021, 13, 13959. [Google Scholar] [CrossRef]
- Aranda Sagredo, C. Plan maestro de recualificación sostenible en Laguna Verde, in Facultad de Arquitectura y Urbanismo. Bachelor´s Thesis, Universidad de Chile, Santiago, Chile, 2013. [Google Scholar]
- Wang, M.; Gong, H. Imbalanced Development and Economic Burden for Urban and Rural Wastewater Treatment in China—Discharge Limit Legislation. Sustainability 2018, 10, 2597. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.; Ader, D. The Demography of Rural Latin America: The Case of Chile, in International Handbook of Rural Demography; Springer: Berlin/Heidelberg, Germany, 2012; pp. 95–110. [Google Scholar]













| Gene | Group | Primers | Primer Concentration (µM) | Annealing Temperature (°C) | Efficiency (%) | R2 | Reference |
|---|---|---|---|---|---|---|---|
| Ammonia monooxygenase subunit A (amoA) | Betaproteobacteria βAOB | amoA1F | 0.4 | 56 | 92.55% | 0.998 | [43] |
| amoA2R | 0.4 | ||||||
| Ammonia monooxygenase subunit A (comaA) | COMAMMOX Nitrospira Clade A | comaA-244F coma-659R | 0.4 | 52 | 83.10% | 0.999 | [44] |
| 0.4 | |||||||
| Methane monooxygenase subunit A (pmoA) | Methanotrophs | A189F | 0.4 | 55 | 90.34% | 0.999 | [45] |
| mb661R | 0.4 | [46] | |||||
| Methyl coenzyme M reductase subunit A (mcrA) | Methanogens | mlasmcrA-rev | 1 | 55 | 90.59% | 0.999 | [47] |
| 1 |
| WINTER | SUMMER | MAL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FACTOR | F2 | F3 | E7 | E10 | E11 | F2 | F3 | E7 | E10 | E11 | |
| DTS (mg/L) | 381 | 587 | 543 | 563 | 647 | 413 | 645 | 660 | 1406 | >2000 | <500 |
| Transparency (Secchi disk depth in cm) | 18 | 40 | 12 | 60 | 84 | ND | ND | ND | 60 (0.61; 0.2) | 4 (0.11; 0.12) | ≥120 |
| Turbidity (NTU) | 5.54 | 18.53 | 15.60 | 10.02 | 14.27 | 1.93 | 32.83 | 55.40 | 4.91 | 10.16 | 50 |
| Color (Pt/C) | 139.00 | 316.00 | 299.67 | 183.00 | 278.67 | 254.00 (139; 126) | 624.00 (448; 1063) | 715.00 (550; 1572) | 239.00 (415; 75) | 218.00 (350; 225) | <100 |
| Sulfate (mg/L) | 85.33 | 244.00 | 141.33 | 133.33 | 136.00 | 124.85 | 159.15 | 153.66 | 205.80 | 270.28 | 250 |
| Chlorides (mg/L) | 75.63 | 146.53 | 146.53 | 184.34 | 255.24 | 0.70 (70.9; 113.5) | 1.43 (106. 4; 156) | 1.57 (355; 269) | 5.30 (142; 326) | 8.73 (709; 2822) | 200 |
| Total Coliforms (NMP) | 9200 | <1.8 | <1.8 | ND | 1100 | ≥16,000 (>16,000; >1600) | 0 (>16,000; <1.8) | 110 | 500 | 2200 | 1000 |
| Fecal Coliforms (NMP) | 140 | <1.8 | <1.8 | ND | 490 | 260 (<1.8; 20) | 0 (20; <1.8) | 80 | 300 | 140 | 1000 |
| BOD5 (mg/L) | 7.30 | 0.00 | 4.35 | 4.51 | 11.98 | 11.3 (1000; 5.44) | 65.79 (1400; 99.9) | 66.41 | 17.71 | 20.31 | 20 |
| Chlorophyll-a (mg/L) | 6.52 | 0.6 | 0.19 | 20.02 | 41.14 | 31.81 | 0.39 | 39.28 | 22.74 | 32.92 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozo-Solar, F.; Cornejo-D’Ottone, M.; Orellana, R.; Acuña, C.; Rivera, C.; Aguilar-Muñoz, P.; Lavergne, C.; Molina, V. Microbial and Biogeochemical Shifts in a Highly Anthropogenically Impacted Estuary (“El Sauce” Valparaíso). Water 2023, 15, 1251. https://doi.org/10.3390/w15061251
Pozo-Solar F, Cornejo-D’Ottone M, Orellana R, Acuña C, Rivera C, Aguilar-Muñoz P, Lavergne C, Molina V. Microbial and Biogeochemical Shifts in a Highly Anthropogenically Impacted Estuary (“El Sauce” Valparaíso). Water. 2023; 15(6):1251. https://doi.org/10.3390/w15061251
Chicago/Turabian StylePozo-Solar, Francisco, Marcela Cornejo-D’Ottone, Roberto Orellana, Carla Acuña, Cecilia Rivera, Polette Aguilar-Muñoz, Céline Lavergne, and Verónica Molina. 2023. "Microbial and Biogeochemical Shifts in a Highly Anthropogenically Impacted Estuary (“El Sauce” Valparaíso)" Water 15, no. 6: 1251. https://doi.org/10.3390/w15061251