Polyethylene Microplastic Particles Alter the Nature, Bacterial Community and Metabolite Profile of Reed Rhizosphere Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Description
2.3. Observation of Surface Morphology Changes of LDPE Microplastics
2.4. Determination of the Physicochemical Properties of Reed Rhizosphere Soil in Each Treatment Group
2.5. Determination of Reed Rhizosphere Soil Enzyme Activity in Each Treatment Group
2.6. Analysis of Microbial Diversity in Reed Rhizosphere Soil
2.7. Analysis of Metabolic Profiling in Reed Rhizosphere Soil
2.8. Data Statistics and Analysis
3. Results
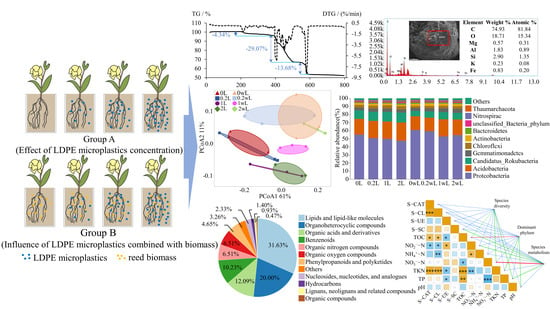
3.1. Effects of LDPE Microplastic Addition and Reed Biomass on Soil Physical and Chemical Properties in Rhizosphere
3.2. Surface Morphology Changes of LDPE Microplastics
3.3. Distribution of Soil Elements on the Surface of LDPE Microplastics
3.4. The Effects of Microplastics on Soil Enzyme Activity
3.5. Effect of LDPE Microplastics on the Rhizosphere Soil Microbial Community
3.6. Effects of LDPE Microplastics and Reed Biomass on Rhizosphere Soil Metabolism
3.7. Correlation Analysis of Physicochemical Properties and Soil Community Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Colton, J.B.; Burns, B.R.; Knapp, F.D. Plastic Particles in Surface Waters of the Northwestern Atlantic. Science 1974, 185, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Besseling, E.; Foekema, E.; Kooi, M.; Mintenig, S.; Ossendorp, B.C.; Redondo-Hasselerharm, P.E.; Verschoor, A.; van Wezel, A.P.; Scheffer, M. Risks of Plastic Debris: Unravelling Fact, Opinion, Perception, and Belief. Environ. Sci. Technol. 2017, 51, 11513–11519. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Bary, A.I.; Hayes, D.G.; Wadsworth, L.C.; Anunciado, M.B.; English, M.E.; Bandopadhyay, S.; Schaeffer, S.M.; DeBruyn, J.M.; Miles, C.A.; et al. In situ degradation of biodegradable plastic mulch films in compost and agricultural soils. Sci. Total Environ. 2020, 727, 138668. [Google Scholar] [CrossRef]
- Fei, Y.; Huang, S.; Zhang, H.; Tong, Y.; Wen, D.; Xia, X.; Wang, H.; Luo, Y.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020, 707, 135634. [Google Scholar] [CrossRef]
- Fu, Q.; Lai, J.-L.; Ji, X.-H.; Luo, Z.-X.; Wu, G.; Luo, X.-G. Alterations of the rhizosphere soil microbial community composition and metabolite profiles of Zea mays by polyethylene-particles of different molecular weights. J. Hazard. Mater. 2022, 423, 127062. [Google Scholar] [CrossRef]
- Falsini, S.; Colzi, I.; Chelazzi, D.; Dainelli, M.; Schiff, S.; Papini, A.; Coppi, A.; Gonnelli, C.; Ristori, S. Plastic is in the air: Impact of micro-nanoplastics from airborne pollution on Tillandsia usneoides (L.) L. (Bromeliaceae) as a possible green sensor. J. Hazard. Mater. 2022, 437, 129314. [Google Scholar] [CrossRef]
- Kumar, R.; Ivy, N.; Bhattacharya, S.; Dey, A.; Sharma, P. Coupled effects of microplastics and heavy metals on plants: Uptake, bioaccumulation, and environmental health perspectives. Sci. Total Environ. 2022, 836, 155619. [Google Scholar] [CrossRef]
- Hou, L.; Xi, J.; Liu, J.; Wang, P.; Xu, T.; Liu, T.; Qu, W.; Lin, Y.B. Biodegradability of polyethylene mulching film by two Pseudomonas bacteria and their potential degradation mechanism. Chemosphere 2022, 286, 131758. [Google Scholar] [CrossRef] [PubMed]
- Menossi, M.; Cisneros, M.; Alvarez, V.A.; Casalongué, C. Current and emerging biodegradable mulch films based on polysaccharide bio-composites. A review. Agron. Sustain. Dev. 2021, 41, 53. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E. Effect of (bio)plastics on soil environment: A review. Sci. Total Environ. 2021, 795, 148889. [Google Scholar] [CrossRef]
- Li, J.; Song, Y.; Cai, Y. Focus topics on microplastics in soil: Analytical methods, occurrence, transport, and ecological risks. Environ. Pollut. 2020, 257, 113570. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Ossowicki, A.; Yang, X.; Lwanga, E.H.; Dini-Andreote, F.; Geissen, V.; Garbeva, P. Effects of plastic mulch film residues on wheat rhizosphere and soil properties. J. Hazard. Mater. 2020, 387, 121711. [Google Scholar] [CrossRef]
- Miller, S.B.; Heuberger, A.L.; Broeckling, C.D.; Jahn, C.E. Non-Targeted Metabolomics Reveals Sorghum Rhizosphere-Associated Exudates are Influenced by the Belowground Interaction of Substrate and Sorghum Genotype. Int. J. Mol. Sci. 2020, 20, 431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Shen, J.; Sun, G.; Wang, B.; Ji, R.; Zhao, L. Foliar Application of SiO2 Nanoparticles Alters Soil Metabolite Profiles and Microbial Community Composition in the Pakchoi (Brassica chinensis L.) Rhizosphere Grown in Contaminated Mine Soil. Environ. Sci. Technol. 2020, 54, 13137–13146. [Google Scholar] [CrossRef]
- Wu, C.; Ma, Y.; Wang, D.; Shan, Y.; Song, X.; Hu, H.; Ren, X.; Ma, X.; Cui, J.; Ma, Y. Integrated microbiology and metabolomics analysis reveal plastic mulch film residue affects soil microorganisms and their metabolic functions. J. Hazard. Mater. 2022, 423, 127258. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Y.; Sun, X.; Peng, Y.; Xiao, L. Mixing effect of polylactic acid microplastic and straw residue on soil property and ecological function. Chemosphere 2020, 243, 125271. [Google Scholar] [CrossRef]
- Koskei, K.; Munyasya, A.N.; Wang, Y.; Zhao, Z.; Zhou, R.; Indoshi, S.N.; Wang, W.; Cheruiyot, W.K.; Mburu, D.M.; Nyende, A.B.; et al. Effects of increased plastic film residues on soil properties and crop productivity in agro-ecosystem. J. Hazard. Mater. 2021, 414, 125521. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhou, S.; Zhang, C.; Zhou, Y.; Qin, W. Soil microplastic characteristics and the effects on soil properties and biota: A systematic review and meta-analysis. Environ. Pollut. 2022, 313, 120183. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Yan, B.; Wang, H.; Wu, Y.; Wang, X.; Wang, J.; Zhu, Z.; Yan, X.; Liu, Y.; Liu, M.; et al. Effects of microplastics on nitrogen and phosphorus cycles and microbial communities in sediments. Environ. Pollut. 2023, 318, 120852. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jin, T.; Zou, T.; Xu, L.; Xi, B.; Xu, D.; He, J.; Xiong, L.; Tang, C.; Peng, J.; et al. Current progress on plastic/microplastic degradation: Fact influences and mechanism. Environ. Pollut. 2022, 304, 119159. [Google Scholar] [CrossRef] [PubMed]
- Camacho, M.; Herrera, A.; Gómez, M.; Acosta-Dacal, A.; Martínez, I.; Henríquez-Hernández, L.A.; Luzardo, O.P. Organic pollutants in marine plastic debris from Canary Islands beaches. Sci. Total Environ. 2019, 662, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Mahfooz, Y.; Yasar, A.; Guijian, L.; Islam, Q.U.; Akhtar, A.B.T.; Rasheed, R.; Irshad, S.; Naeem, U. Critical risk analysis of metals toxicity in wastewater irrigated soil and crops: A study of a semi-arid developing region. Sci. Rep. 2020, 10, 12845. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Li, Y.; Li, J.; Liu, Y.; Xia, S.; Zhao, J. Effects of exposure of polyethylene microplastics to air, water and soil on their adsorption behaviors for copper and tetracycline. Chem. Eng. J. 2021, 404, 126412. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere 2020, 239, 124792. [Google Scholar] [CrossRef]
- Lu, S.; Shen, L.; Li, X.; Yu, B.; Ding, J.; Gao, P.; Zhang, H. Advances in the photocatalytic reduction functions of graphitic carbon nitride-based photocatalysts in environmental applications: A review. J. Clean. Prod. 2022, 378, 134589. [Google Scholar] [CrossRef]
- Yu, B.; Li, X.; He, M.; Li, Y.; Ding, J.; Zhong, Y.; Zhang, H. Selective production of singlet oxygen for harmful cyanobacteria inactivation and cyanotoxins degradation: Efficiency and mechanisms. J. Hazard. Mater. 2023, 441, 129940. [Google Scholar] [CrossRef]
- Li, X.; Qu, C.; Bian, Y.; Gu, C.; Jiang, X.; Song, Y. New insights into the responses of soil microorganisms to polycyclic aromatic hydrocarbon stress by combining enzyme activity and sequencing analysis with metabolomics. Environ. Pollut. 2019, 255, 113312. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Yang, X.; Riksen, M.; Geissen, V. Effect of different polymers of microplastics on soil organic carbon and nitrogen—A mesocosm experiment. Environ. Res. 2022, 204, 111938. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic Stoichiometry and Ecological Theory. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 313–343. [Google Scholar] [CrossRef] [Green Version]
- Bowles, T.M.; Acosta-Martínez, V.; Calderón, F.; Jackson, L.E. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an intensively-managed agricultural landscape. Soil Biol. Bio-Chem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Ya, H.; Xing, Y.; Zhang, T.; Lv, M.; Jiang, B. LDPE microplastics affect soil microbial community and form a unique plastisphere on microplastics. Appl. Soil Ecol. 2022, 180, 104623. [Google Scholar] [CrossRef]
- Lee, S.-H.; Sorensen, J.; Grady, K.L.; Tobin, T.C.; Shade, A. Divergent extremes but convergent recovery of bacterial and archaeal soil communities to an ongoing subterranean coal mine fire. ISME J. 2017, 11, 1447–1459. [Google Scholar] [CrossRef]
- Li, C.; Cui, Q.; Li, Y.; Zhang, K.; Lu, X.; Zhang, Y. Effect of LDPE and biodegradable PBAT primary microplastics on bacterial community after four months of soil incubation. J. Hazard. Mater. 2022, 429, 128353. [Google Scholar] [CrossRef]
- Luo, G.; Jin, T.; Zhang, H.; Peng, J.; Zuo, N.; Huang, Y.; Han, Y.; Tian, C.; Yang, Y.; Peng, K.; et al. Deciphering the diversity and functions of plastisphere bacterial communities in plastic-mulching croplands of subtropical China. J. Hazard. Mater. 2022, 422, 126865. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, C.; Cao, N.; Li, X.; Li, X.; Chen, Y.; Huang, Y.; Wang, J. Effects of microplastics on soil microbiome: The impacts of polymer type, shape, and concentration. Sci. Total Environ. 2022, 806, 150516. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Zhu, Q.; Jin, T.; Peng, J.; Zuo, N.; Zhang, H.; Geng, M.; Huang, Y.; Han, Y.; Tian, C.; et al. Crop types and irrigation regimes as drivers of plastisphere bacterial communities in plastic-mulching croplands of subtropical China. Appl. Soil Ecol. 2023, 182, 104696. [Google Scholar] [CrossRef]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, T.; Li, J.; Liu, C. Improvement of α-amylase to the metabolism adaptions of soil bacteria against PFOS exposure. Ecotoxicol. Environ. Saf. 2021, 208, 111770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, M.; Zhang, W.; Gardea-Torresdey, J.L.; White, J.C.; Ji, R.; Zhao, L. Silver Nanoparticles Alter Soil Microbial Community Compositions and Metabolite Profiles in Unplanted and Cucumber-Planted Soils. Environ. Sci. Technol. 2020, 54, 3334–3342. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, J.; He, Y.; Yu, X.; Chen, S.; Penttinen, P.; Liu, S.; Yang, Y.; Zhao, K.; Zou, L. Organic Fertilizers Shape Soil Microbial Communities and Increase Soil Amino Acid Metabolites Content in a Blueberry Orchard. Microb. Ecol. 2022, 85, 232–246. [Google Scholar] [CrossRef]
- Li, Z. The Chemistry and Histology of Animal Skins; China Light Industry Press: Beijing, China, 2010. [Google Scholar]






| Reed Biomass Dose | 0% | 2% | |
|---|---|---|---|
| LDPE Microplastic Dose | (Group A) | (Group B) | |
| 0% | 0 L | 0 wL | |
| 0.2% | 0.2 L | 0.2 wL | |
| 1% | 1 L | 1 wL | |
| 2% | 2 L | 2 wL | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Liu, B.; Zhang, W.; Liang, F.; Wu, J.; Song, Z.; Zhu, Y. Polyethylene Microplastic Particles Alter the Nature, Bacterial Community and Metabolite Profile of Reed Rhizosphere Soils. Water 2023, 15, 1505. https://doi.org/10.3390/w15081505
Tian Z, Liu B, Zhang W, Liang F, Wu J, Song Z, Zhu Y. Polyethylene Microplastic Particles Alter the Nature, Bacterial Community and Metabolite Profile of Reed Rhizosphere Soils. Water. 2023; 15(8):1505. https://doi.org/10.3390/w15081505
Chicago/Turabian StyleTian, Zeyuan, Biao Liu, Wenjun Zhang, Fan Liang, Junfeng Wu, Zhongxian Song, and Yichun Zhu. 2023. "Polyethylene Microplastic Particles Alter the Nature, Bacterial Community and Metabolite Profile of Reed Rhizosphere Soils" Water 15, no. 8: 1505. https://doi.org/10.3390/w15081505