.tmb-768v.png?sfvrsn=d6990130_3)
C-TAP – A pioneering approach to enhance the global production of and access to COVID-19 health products through transparent, voluntary, non-exclusive licensing
A global one-stop shop for COVID-19 health products
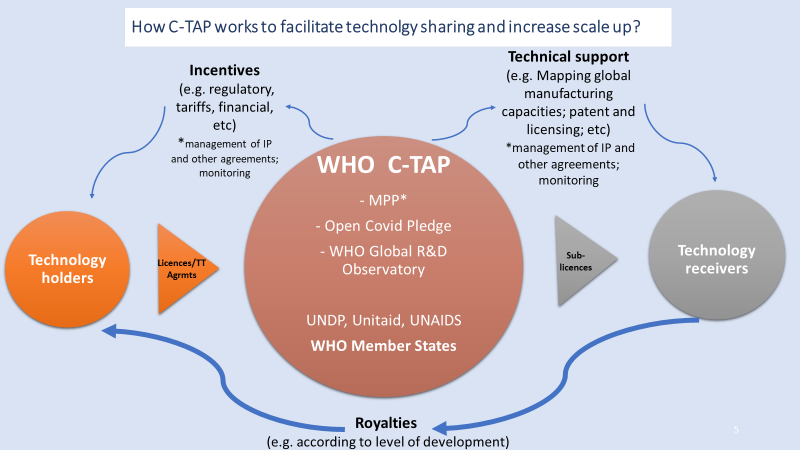
The COVID-19 Technology Access Pool (C-TAP) is a single platform for the developers of COVID-19 health products – vaccines, tests, medical devices and treatments – to share their know-how, intellectual property (IP), and data with quality-assured manufacturers in order to boost the global supply and accelerate the end to the pandemic.
C-TAP, so far endorsed by nearly 45 Member States, is based on public health-oriented, transparent, voluntary, and non-exclusive licences, which could be issued through the Medicines Patent Pool, a C-TAP partner.
Such licences aim to provide the qualified manufacturers with:
- The legal rights to manufacture and sell the licensed products;
- The technology and know-how required to develop quality-assured products effectively and efficiently;
- Access to clinical data needed to obtain regulatory approval for their products.
A win-win solution for all
- People in low- and middle-income countries gain faster access to the desperately needed COVID-19 health products.
- Developers of COVID-19 vaccines and other health products use C-TAP to reach new markets, access the untapped production capacity and secure appropriate royalties for the use of their IP, know-how, and data.
- With support and technical assistance provided through WHO and other C-TAP partners, many more manufacturers around the world begin producing lifesaving COVID-19 vaccines and other products that meet international standards of quality, safety, and efficacy.
- Countries benefit from increased availability and affordability of COVID-19 health products, allowing them to utilise savings for other healthcare needs.
The promise that C-TAP holds: fast-tracking access, availability and affordability of COVID-19 health products for all
- A speedy solution within the existing IP system that leverages the proven model of voluntary licensing through the longstanding experience of partners like MPP and world-class technical expertise of WHO and other UN agencies.
- An all-inclusive mechanism that will facilitate access to patents, as well as broader technology, know-how and clinical trial data needed by manufacturers for efficient production and regulatory approvals.
- Gives IP holders a fair deal through royalties and IP management support.
- Ensures that products adhere to quality standards set by the WHO.
- Returns public investment to the public by speeding up equitable access to COVID-19 health products and facilitating a full global recovery.
- Creates stronger, self-sustaining global manufacturing systems, especially in low- and middle-income countries. This is essential not only to tackle the pandemic at hand but also to address future public health emergencies.
Standing for equitable access to COVID-19 health products and supporting C-TAP
- Governments and other research funders can encourage originator companies to share their technologies in a voluntary, non-exclusive and transparent way with C-TAP.
- Pharmaceutical companies and publicly funded research institutes that have developed COVID-19 products can promote wider access to these lifesaving health products by voluntarily licensing their technologies to C-TAP, sharing relevant IP, data and facilitating technology transfer.
- Pharmaceutical manufacturers, particularly in low- and middle-income countries, can express their willingness to work with C-TAP and offer their manufacturing capacity.
- Civil society advocates and opinion leaders can help spread the word on the urgent need for equitable access to COVID-19 health products – the mission of C-TAP – and encourage public and private stakeholders to do their part.
