Nomenclature of medical devices
What are medical devices?
Medical devices include all the health technologies (except for vaccines and medicines) required for prevention, diagnosis, treatment, monitoring, rehabilitation and palliation. They are indispensable for universal health coverage, monitoring wellbeing and addressing outbreaks or emergencies. Some types of medical devices include:
- single use devices (i.e. syringes, catheters)
- implantable (i.e. hip prothesis, pacemakers)
- imaging (i.e. ultrasound and CT scanners)
- medical equipment (i.e. anesthesia machines, patient monitors, hemodialysis machines)
- software (i.e. computer aided diagnostics)
- in vitro diagnostics (i.e. glucometer, HIV tests)
- personal protective equipment (i.e. mask, gowns, gloves)
- surgical and laboratory instruments
What is nomenclature for medical devices?
The nomenclature of medical devices is a coding and naming system used to generically classify and identify all medical devices and related health products. According to different classification and nomenclature systems, there are between 5,000 to 24,000 different types of medical devices. They range from the very simple to complex, inexpensive to costly. The number and types of devices are increasing exponentially
Why is there a need for a standardized nomenclature for medical devices?
The multiple nomenclatures in existence makes it difficult to communicate important information between individuals and organizations, which can result in health, economic and social impact. It complicates procurement, supply and trade, and tracking of medical devices. Some important information negatively impacted by an absence of a standardized nomenclature include:
- patient safety
- intended use of medical devices
- regulatory status
- technical information
- adverse events
- availability
- others
A standardized classification and nomenclature of medical devices will serve as a common language for recording and reporting medical devices across the whole health system, at all levels of health care, and for a whole range of uses. Therefore, WHO is working towards a globally accessible, transparent and harmonized nomenclature system.
Standardization of nomenclature is also essential for:
- grouping and evaluating innovative technologies
- classifying for regulatory approval (registration)
- streamlining procurement
- supporting device descriptions for universal health coverage benefits packages
- ordering and grouping of devices in electronic health records and other health information systems
WHO and nomenclature for medical devices
Member States information
Several systems exist today to name and classify medical devices. Each system is used by a different group of professionals depending on the needs of that group. Such needs include: regulatory approval, procurement and supply, customs operation, inventories and maintenance, among others. Some countries and organizations have developed their own national systems that are not compatible across systems.
In 2010 and 2017 WHO conducted surveys asking about Nomenclature systems. As many as 174 countries responded. About half of the responding member states (52%, n=90), use at least one official nomenclature system for medical devices. In contrast, 84 member states did not have any official national nomenclature (49%). More information can be found in the Global Atlas of Medical Devices (2017). The GAMD 2022 (including information by country on the use of nomenclature systems for medical devices), was open for consultation until December 2021. Up-dates on nomenclature systems for medical devices from member state's Ministries of Health have been included. Please find further information in the WHO GAMD website.

Global atlas of medical devices 2022
Member States requests
In resolution WHA60.29 (2007), WHO was requested to work with interested Member States and WHO collaborating centres on the development of guidelines and tools relating to health technologies (in particular medical devices). Such guidelines and tools were to include norms, standards and a standardized glossary of definitions. All stakeholders were to use a transparent and evidence-based process.
WHO standardized nomenclature for medical devices
A standardized international classification coding and nomenclature of medical devices could link to WHO’s other international classification systems, such as the International Statistical Classification of Diseases and Related Health Problems, the International Classification of Functioning, Disability and Health and for health products, the International Non-proprietary Name for medicines, in order to support organized and standardized information for policy-makers and managers. In March 2011 WHO convened representatives from the nomenclature agencies to search for convergence in Nomenclature of Medical Devices which has not been achieved to date.
WHO consultations on nomenclature for medical devices
Since 2010, WHO has conducted several consultations on issues related to medical devices, including discussions during WHO’s past Global Fora on medical devices (in 2010, 2013, 2017 and 2018), and country surveys to determine country needs. WHO has also developed guidance documents on: how to formulate policies on medical devices; how to select, assess, manage and procure relevant devices; and effective donation of medical devices. In all the above-mentioned Fora, documents or consultations, the topic of lack of standardized nomenclature was noted.
WHO request for collaboration on nomenclature for medical devices
In 2018, WHO published a Request for input and collaboration towards international classification, coding and nomenclature of medical devices, that included the principles of governance, classification and access of information. WHO received 43 comments from Member States and other organizations. Since then, WHO has been searching for a solution that complies with those principles, which are summarized below.
Governance
- Organizational and review structures should be in place to ensure that all stakeholders from different regions are able to provide feedback according to global needs.
Classification, coding and nomenclature characteristics
The following are required:
- a transparent methodology and processes;
- a transparent mechanism for regular updates (e.g. once per year);
- hierarchies grouped into categories and subcategories to meet stakeholder needs;
- medical devices used outside highly regulated countries;
- mutually exclusive terms;
- availability of terms in other languages
Access to information
Information should:
- be capable of being referenced and used by regulators, procurers, managers and all users of medical devices (hospitals/health care workers and patients);
- be freely available and considered a global public good;
- support unique device identifier system;
- be accessible through simple and intuitive search;
- be available for use in all health-related data base systems.
Which characteristics are required for a standardized nomenclature for medical devices?
Countries and other stakeholders need a globally accessible, transparent, and harmonized nomenclature system for medical devices that adhere to WHO principles. The lack of such a system is impeding progress toward access to medical devices, which has a negative impact on efforts to facilitate emergency interventions and, more broadly, achieve universal health coverage.
2019-2021 WHO Executive Board sessions related to a standardized nomenclature for medical devices
- On 29 May 2019 during the Executive Board 145 the report Standardization of medical devices nomenclature EB145/3 was presented on recommendations to have an international classification, coding and nomenclature for medical devices. The Nomenclature system would be available to all Member States and would support: patient safety, access to medical devices for universal health coverage, emergency support preparedness and response for emergencies, efforts to increase quality of healthcare, and achievement of Sustainable Development Goal 3. The Executive Board webcast presents the statements by Member States (Agenda Item 5)
- On 23 January 2021, WHO Executive Board 148 discussed the EB148/13 Standardized Nomenclature of Medical Devices Report. The report proposes that WHO will neither develop a new nomenclature of medical devices nor adopt a proprietary system but harmonize with the European Medical Device Nomenclature, which will support regulation, assessment and management of medical devices to improve access. Statements by Member States are available on the webcast Executive Board 148.
- Member States required information and consultation sessions. The first session for Member States, will take place 12 of April 2021, and additional meetings will be convened with other stakeholders.
- In April 2021 WHO updated the Analysis of Existing Nomenclatures of Medical Devices as requested by Member States. When more information becomes available the analysis will be updated.

2021 Consultations and information sessions after Executive Board 148/13
2021-2022 Calendar of events
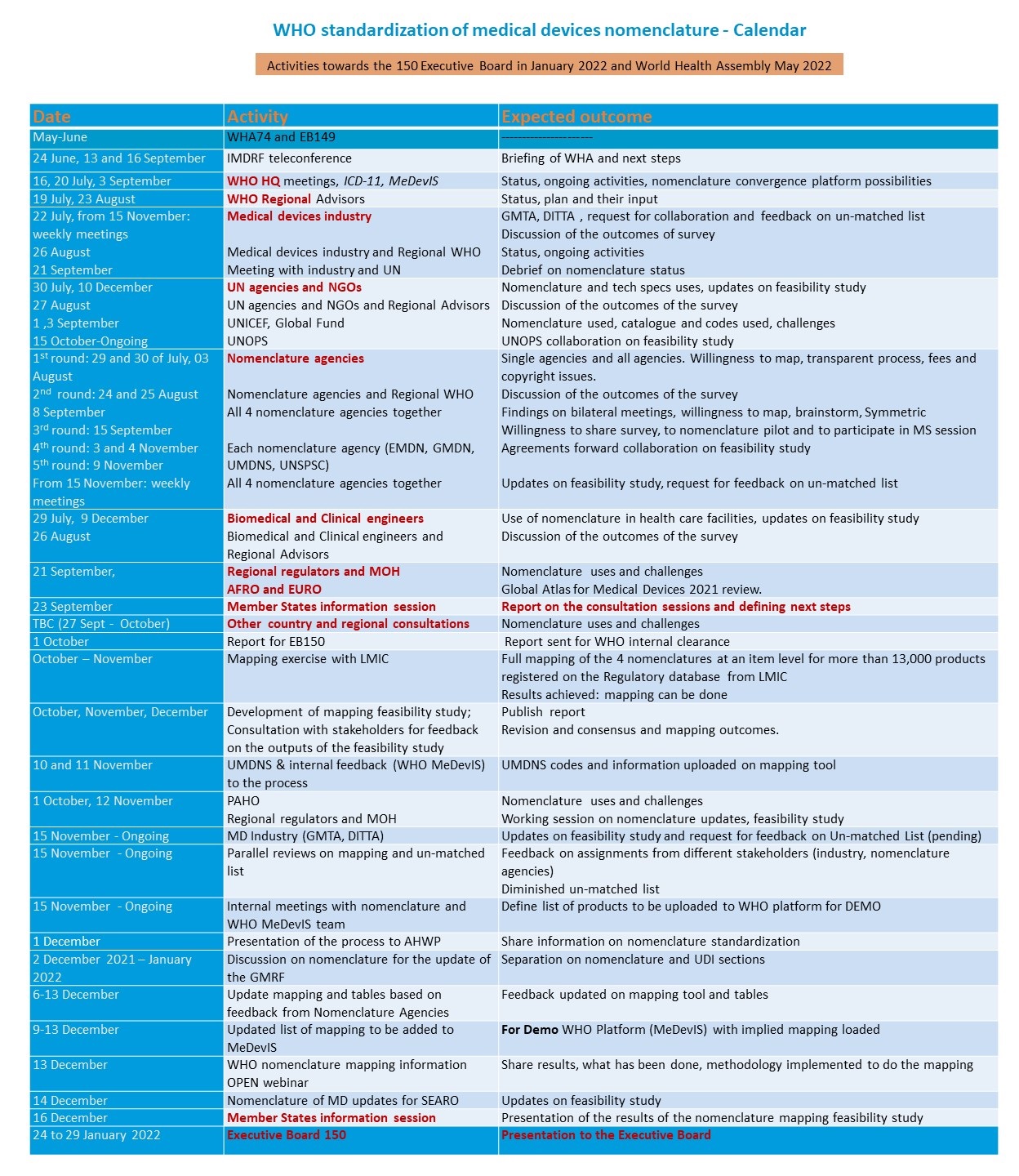
2021 Member States information sessions
Committee A
Friday 28 May 2021 from 15:00 to 17.00
Saturday 29 May 2021 from 10:00 to 12:00
WHO Seventy-fourth World Health Assembly discussion on the nomenclature of medical devices
- Even though there is a general agreement that a standardized nomenclature should be available for all Member States, as an element to support the assessment, regulations, procurement and management of medical devices, the discussions have been polarized: a set of countries advocates for -a proprietary system- and a set of countries do not agree with this proposal and have requested WHO to advise on a relevant approach taking in to account existing systems.
- As requested by Member States during the 145th session of the Executive Board, WHO will not be creating a new nomenclature, but will select from the available ones to work towards convergence.
- WHO Secretariat was requested at the EB in January to hold information sessions with MS -one took place on 12 April with all Member States, and 3 other meetings with: Andean countries, European Commission Medical devices consultation Group and with NGOs approved by FENSA and Biomedical engineers. And presented to the IMDRF.
- More information sessions and consultations will take place from June to September 2021 with stakeholders: regulators, industry, procurers and health facility managers, and Member States.
- WHO expects to have a new report in October with the outcomes of surveys and consultations and have it ready to be presented for discussion for the next EB 150.
- WHO requires help from MS, to find agreements between available systems, including between the Global medical devices nomenclature proprietary system, the European Medical Devices Nomenclature, and other major nomenclature systems, to ensure an international classification, coding and nomenclature is available, especially in these times when access to medical devices is indispensable for the outbreak response and to reinforce health systems.
July - October 2021 - WHO consultation on nomenclature systems for medical devices
September-October 2021 - Overview of Nomenclature Systems for
Medical Devices in WHO Member
States. 2021 Country Consultation and Desk Review
During 2021 WHO conducted a country consultation and desk review centered on Member States use of medical devices nomenclature systems. The complete document was available for review until December 2021. Now the consultation is closed. The final document will be available in 2022.
December 2021 - Mapping of nomenclature for medical devices a feasibility study
Methodology Overview
| 1. | Download records from AccessGUDID and Italian Ministry of Health (surrogate for EUDAMED) |
| 2. | Match records with the same UDI-DI in both databases |
| 3. | Map GMDN terms in AccessGUDID to most recently published GMDN version to get GMDN codes |
| 4. | Map CND codes in Italian Ministry of Health to the most recentl published EMDN version |
| 5. | These items are the full set of items that appear in both databases |
| 6. | Narrow the overlapping items to COVID19, implants and commodities - important to WHO initiatives |
| 7. | Reduce the number of items to between 10,000-15,000 to show value of automation without overwhelming reviewers |
| 8. | Assign UNSPSC and UMDNS codes/terms to selected items |
| 9. | Integrate all mappings (see Mapping of 13,129 items) |
| 10. | Roll up item level mapping into groups for use by MeDevIS (version with the nomenclature mapping) |
| 11. | Automatically upload mapping into MeDevIS |
| 12. | MeDevIS team evaluates mappings to approve or reject results |
Optional: Use Machine Learning models to flag items for expert review:
| A. | Apply ML Model to identify nomenclature assignments to UDI-DI where nomenclature assignment and manufacturer description appear dissimilar |
| B. | Apply ML model to identify nomenclature assignments to UDI-DI where nomenclature terms appear to be dissimilar |
| C. | Create a statistical distribution of item category assignments showing similar to dissimilar |
| D. | Combine ML scoring of A, B, and C to highlight items for review (see overview of reviewed items) and improve mapping results |
| E. | Receive human expert review feedback & incorporate into mappings |
| F. | Provide human expert reviewed records to ML model to improve model performance |
Summary of Feasibility Study Results
Machine learning models benefit from expert review. The following summarizes number of items flagged for review by the Study ML model Nomenclature assignments to review % of Sample Size
- EMDN 208 devices 1.6%
- GMDN 61 devices 0.5%
- UMDNS 550 devices 4.2%
- UNSPSC 673 devices 5.1%
Nomenclature agencies reviewed these results and provided feedback. This feedback was not used to inform initial testing of the mappings by MeDevIS or the LMIC but can be use with feedback from other experts to improve future nomenclature assignments and mapping results.
Feasibility Study Mapping of 13,129 items
The Feasibility Study Mapping of 13,129 items are available as a spreadsheet upon request. Please send an email to medicaldevices@who.int with the subject: Spreadsheet request nomenclature feasibility study.
24 to 29 January 2022 - Executive board 150 (EB 150)
The standardization of nomenclature was discussed during the WHO Executive Board 150 on 29 January 2022, session 12, 9:10-12:30 am, Item 14.
The documents related to the Executive Board 150 can be found here.
The item about nomenclature of medical devices includes 3 documents:
- EB150/14 This report covers work done by WHO between June to September 2021.Standardization of medical devices nomenclatureInternational classification, coding and nomenclature of medical devices
- EB150/14 Add.1 This report covers from September to December 2021.Standardization of medical devices nomenclatureInternational classification, coding and nomenclature of medical devices
- EB150/14 Add.2 Financial and administrative implications for the Secretariat of decisions proposed for adoption by the Executive Board

February-May 2022
Member States informal consultations
Following the decision of Executive board 150, three Member States information session were arranged before the Seventy-fifth World Health Assembly 22-28 May 2022.
- 31 March 2022, 9:00-10:30 CET
- 29 April 2022, 12:00-15:00 CET
- 12 May 2022, 14:30-17:00 CET
Details for the information session are available in WHO informal list of intergovernmental meetings website.
Nomenclature pilot mapping was integrated in a WHO platform
The outcome to the nomenclature mapping pilot was integrated to the WHO Priority Medical Devices Information System (MeDevIS) with all nomenclature agencies permission.
To view the outcome please freely navigate the page. Here are some examples but many other devices are available from the pilot completed 2021:
Medical devices integrated in other WHO platforms
The last version of the International Classification of Diseases (IDC-11) , includes disease, health conditions, health interventions and some medical devices. Kindly use the examples below to start navigating the platform.
- Stethoscopes, Binaural (pilot project)
- Ventilators in the ICD page with external reference to EMDN, ( pilot project)
Extraordinary review of the global data on medical devices
It came to WHO's attention that some Member States needed to review the information of the 2022 Global Atlas of Medical Devices, which was under consultation in 2021.
An extraordinary extension was given for Member States until 16 May 2022.
Review was only open for official comments from Ministries of Health or National Health Authorities.
The consultation is now closed. WHO received updates from 43 countries (1 AFR, 11 AMR, 29 EUR, 0 EMR, 1 SEAR, 1 WPR). Please find below the updated pie chart depicting existence and type of official nomenclature of medical devices by country.
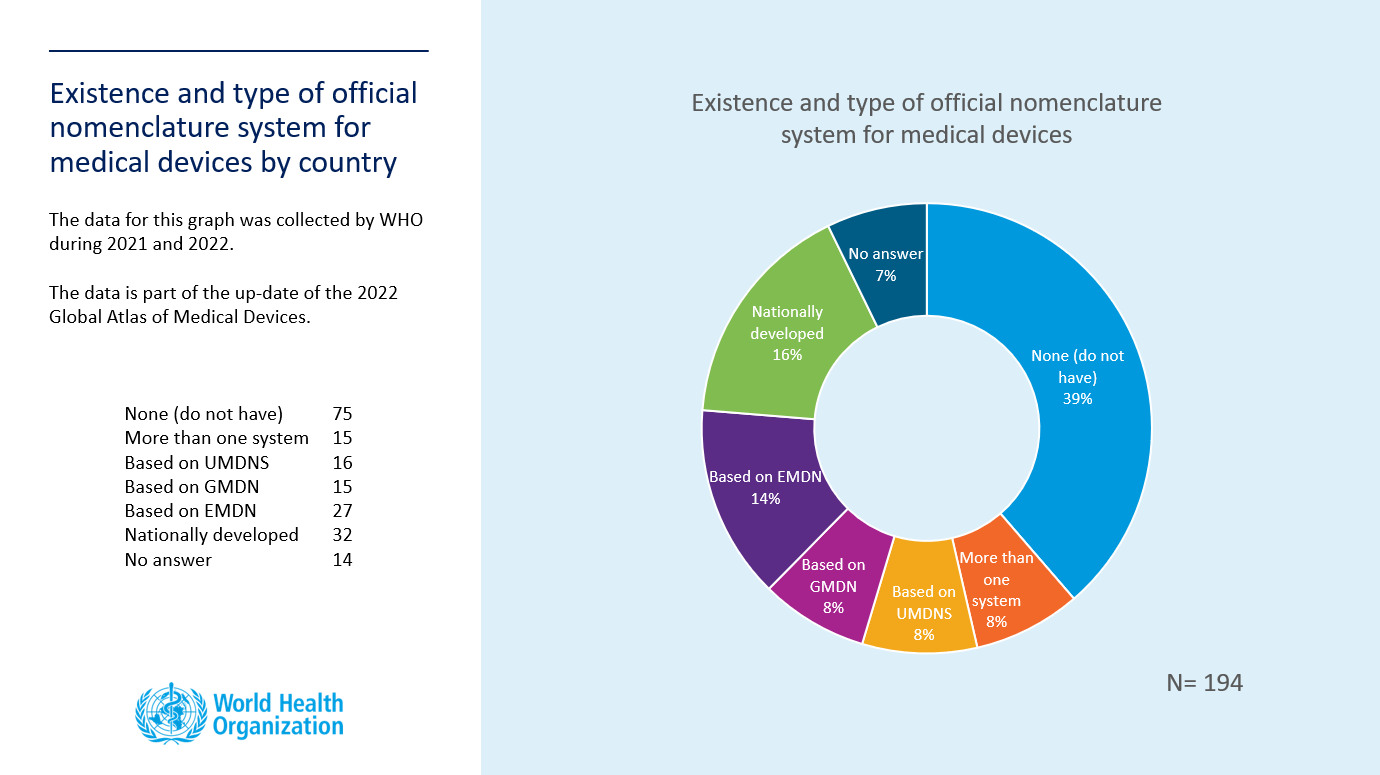
The 2022 Global Atlas of Medical Devices is undergoing publication clearance and will be published in 2022.
22 to 28 May 2022 - Seventy-fifth World Health Assembly (WHA 75)
Decision by WHA75, Item 14.8 Standardization of medical devices nomenclature
Watch WHA75 sessions recording and access all related documents.
The two documents related to nomenclature of medical devices included: A75/11 (the report), and the A75/11 Add1. (the addendum). This last one includes the draft proposals for the decision taken.
A75/11- Standardization of medical devices nomenclatureInternational classification, coding and nomenclature of medical devices
A75/11 Add.1- Standardization of medical devices nomenclature
International classification, coding and nomenclature of medical devices.

The High-Level Welcome event at the opening of the Seventy-fifth World Health Assembly in Geneva, Switzerland on 22 May 2022.
Integration of the mapping of medical devices nomenclature data in WHO platforms
A request for proposal was published in the United Nations Global Market Place from 26 September to 20 October 2022. The purpose of the request was to enter into a contractual agreement with a successful bidder and select a suitable contractor for the provision of mapping medical devices nomenclature data for integration in WHO platforms as follows:
•4,000 types in 5 months.
Six proposals were reviewed including contractors based in 4 different countries. Symmetric Health Solutions was selected to complete the request from December 2022 to July 2023.
/in-page-highlights/database-illustration-1.tmb-1920v.png?sfvrsn=53eec850_2)
2022 Member States information session
30 January to 7 February 2023 - Executive board 152 (EB 152)
The standardization of nomenclature was discussed during the WHO Executive Board 152, Item 11.
Executive Board 152 documentation
The item about nomenclature of medical devices includes 1 document:
- EB152/11 Standardization of medical devices nomenclature International classification, coding and nomenclature of medical devices Report by the Director-General. This report covers work done by WHO between May to September 2022.

21 to 30 May 2023 - World Health Assembly 76 (WHA76)
The standardization of nomenclature was discussed during the World Health Assembly 76/7, Item 13.7
The item about the nomenclature of medical devices includes 1 document:
- A76/7 Rev.1 Standardization of medical devices nomenclature (13.7) .
Decision WHA75(25) Standardization of medical devices nomenclature.
Previously, during the 152 Executive Board in January 2023, a report was presented: EB152/11.
In May 2023, a decision referring to medical devices codes, terms, and definitions was taken: WHA75(25).
The webcast discussion by Member States is available for everyone. Please select: WHA76 - Committee A, twelfth session, on 27/05/2023 - 09:15-11:30 that includes Member States interventions for 13.7 - Standardization of medical devices nomenclature
As the Assistant Director General Dr. Nakatani responded, the secretariat is working on this mandate, and more information and consultations will be available in the following months.

A discussion on poliomyelitis eradication, polio transition planning and polio post-certification at Committee A during the 76th World Health Assembly in Geneva, Switzerland, on 25 May 2023.
2024 Member States information session
